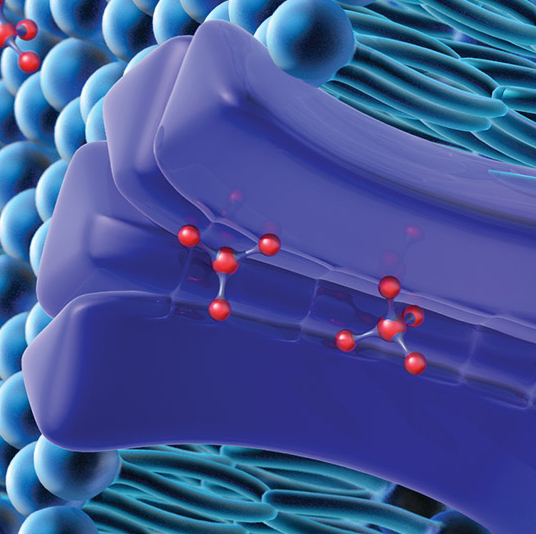
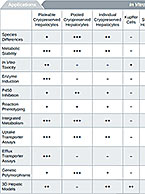
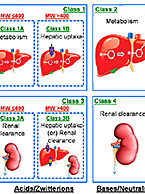
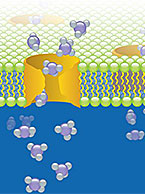
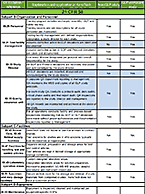
Drug transport studies use regulatory guidance-compliant test systems to investigate a compound’s potential to operate as a substrate or inhibitor of known uptake (SLC) and efflux (ABC) transporters, precipitating a potential drug-drug interaction. For more information about these services, visit our Drug Transport contract service page.