

Why You Need QWBA for Human Radiolabeled ADME Studies
- Regulatory Guidance
- October 4, 2019
- Satoshi Ito
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1.
Quantitative whole body autoradiography (QWBA) is an in vivo study in which a rat (or in some cases mouse) is dosed with radiolabeled test article and at successive time points radioactivity is measured in cross-sectioned slides of the whole animal to show distribution over time.
This study, in addition to data from a rodent mass balance study, provides critical exposure inputs for dosimetry calculations, which are required for planning radiolabeled mass balance studies in human absorption, metabolism, and excretion (hAME) studies. Regulatory agencies mandate the use of radiolabeled drugs to identify and profile human drug metabolism, and dosimetry (exposure) calculations to provide “reasonable prediction of the radiation absorbed by human volunteers”2 to address regulatory requirements that radioactivity from the radiolabeled drug will not harm human subjects3. Data from subsequent human mass balance and ADME studies are required for filing a New Drug Application (NDA) to get a drug approved for market.
Dosimetry calculations require rodent QWBA (distribution) data from pigmented rats (for consideration of melanin binding) as well as mass balance (excretion) data to account for 95% of administered dose. Study designs are to match the sex and route of administration of patients in clinical trials. Even if there is no melanin binding observed, pigmented rats are required unless otherwise agreed upon with the FDA. Animals are treated typically with 100-200 µCi/kg of radiolabeled compound, and one subject is frozen at sacrifice in a CMC block at each of the 5-7 predetermined time points (as informed by earlier pharmacokinetics (PK) studies) over 168 hours, or longer if there is melanin binding.
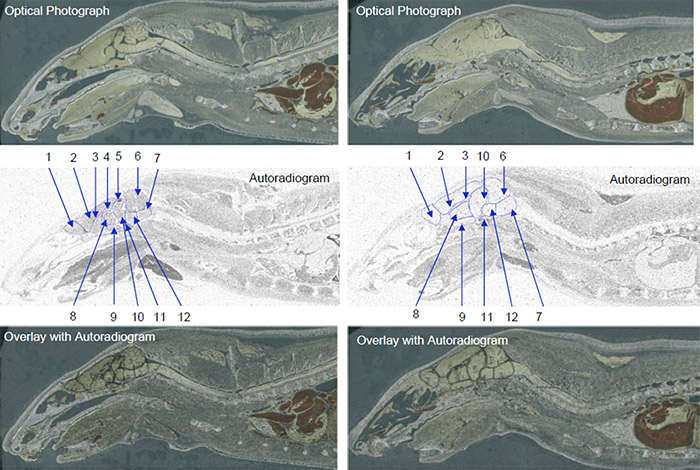
Data obtained from QWBA studies are also applicable to other important PK considerations, i.e. distribution. For example, a QWBA study can tell you how a compound is distributed to both the targeted and non-targeted tissues as well as accumulation in specific parts of an organ (for example, substructures in the brain as pictured in Figure 1).
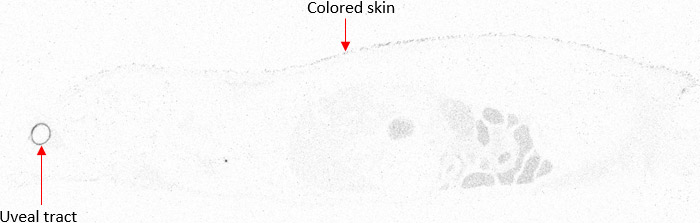
In a typical study package for QWBA, both albino and pigmented rats are used. Pigmented rats, such as Long-Evans, are necessary for the dosimetry calculation inputs, while albino rats such as Sprague-Dawley are selected to match the animals used in toxicity studies. Long-Evans pigmented rats are used to inform on melanin binding by accumulation in skin and uveal tract (Figure 2) which contain high levels of melanin and are required by the FDA for dosimetry input data. By including animals of the tox species as well, e.g. Sprague-Dawley rats, distribution data can be more closely correlated to earlier toxicity data.
At each time point, one animal is sectioned into 30 µM slices at variable depths and each section is imaged two different ways. First, a photograph is taken of a section, to serve as a reference. The slice, a calibration sample, QC sample, and samples for thickness confirmation are placed in contact with an imaging plate (Figure 3). The plate is exposed for a designated exposure period in a sealed lead box and then scanned with a bioimaging analyzer. The resulting radioluminograms are analyzed and the radioactivity in tissues is quantified against the calibration samples.

Whole body autoradiography is unique in its ability to give a drug developer a whole picture of how their drug travels through the body over time. Using images generated by a QWBA study, you can observe clear localization of radioactivity in tissues and organs to demonstrate distribution at continual time points, quantify radioactivity in targeted tissue to estimate effective dose, and detect radioactivity in unexpected tissues.
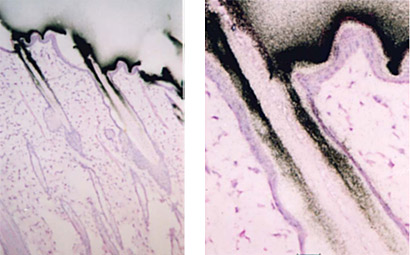
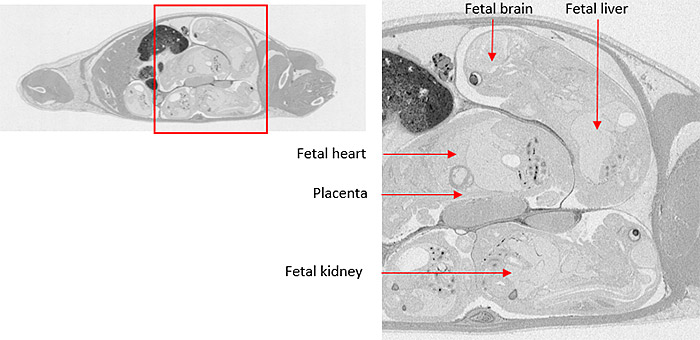
Microautoradiography (mARG) is also available to look more closely at distribution within tissues (Figure 4). Tissues available for mARG include liver, kidney, intestine, tumor, pancreas, skeletal muscle, spleen, retina adrenal gland, and thyroid gland. We also offer options for inclusion of pregnant animals for placental transfer data (seen in Figure 5).
QWBA studies done with the Drug Development Solutions Center provide data to inform:
- Tissue distribution
- Placental transfer
- Melanin binding
- Brain and cerebrospinal fluid exposure
- Estimation of elimination route in various tissues
- Tumor penetration
- Detection of potential sites of drug accumulation
Techniques involved in a QWBA study are highly specialized, and require many hours of experience to prepare slices correctly. Our partners at the Drug Development Solutions Center in Tokai has expertise culminated from more than 50 years in the radioisotope industry. They have the capabilities to work with not only 14C- and 3H-labeled compounds but other isotopes as well to suit peptides, nucleotides, antibodies, and more for in vivo ADME studies. They can also cater to unusual routes of administration, and because their facility is equipped for many different in vivo study types, packages of multiple in vivo studies can be developed to run concurrently all in the same lab to get you consistent, high-quality data faster.
Read More About In Vivo ADME: What You Need and Why You Need It
Visit Our QWBA, Radiolabeling and In Vivo Study Offering Pages
Learn More About Our Other Contract Research Services
Contact Us with Questions or Feedback
Learn more about:
[1] M3 (R2) https://www.fda.gov/media/71542/download
[2] 21CFR312.23 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=361.1
[3]https://www.bioanalysis-zone.com/2018/03/09/quantitative-whole-body-autoradiography-qwba-imaging-mass-spectrometry-ims-can-ims-replace-qwba-support-regulated-drug-discovery-development/
About the Authors
Related Posts
Mass Balance Studies: What You Need and Why You Need It
In Vivo ADME: What You Need and Why You Need It
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
