Author:
FAQ: Using Plateable Hepatocytes in In Vitro Assays
- Test Systems & Methods
- December 13, 2022
- Halee McElhaney, Lucy Fahler
Plateable hepatocytes are often used for induction studies and to research metabolism, biochemistry, virology, host/pathogen interactions, and cell biology. Here,...
Ask Me Anything About In Vitro ADME, Drug-Drug Interaction Approaches and Strategies
- ADME 101™
- December 1, 2022
- Dr. Joanna Barbara
Last month, Dr. Joanna Barbara joined AAPS for an online Q&A in their ongoing “Ask the Experts” series. As regulatory...
Are in vitro drug metabolism and drug-drug interaction studies critical for an IND?
- ADME 101™
- November 1, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith, Amara Millhollen
A Guide to What, Why & When to Conduct ADME Studies When drug developers are preparing their IND, drug metabolism...
4 Ways De-Risking Maximizes Compound Value
- ADME 101™
- August 31, 2022
- Michael Millhollen
Since most new drugs fail because of ADME/Tox, you can add considerable value to your compound by conducting early in...
New FDA Draft Guidance “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics” – XenoTech’s perspective on in vitro DDI testing
- Regulatory Guidance
- July 11, 2022
- Dr. Maciej Czerwinski, Dr. Pallavi Limaye, Dr. Brian Ogilvie
The FDA has released a new draft guidance for industry titled “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics.”...
Spotlight on Efflux and Uptake Drug Transporters in In Vitro Drug-Drug Interaction Studies
- Drug Transporters
- June 23, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith
What are drug transporters? Drug transporters are membrane-bound proteins that assist in the movement of drugs into or out of...
When, Why and How to Conduct CYP2C Induction Studies
- Enzyme Induction
- April 8, 2022
- Dr. Andrew G. Taylor, Rebecca Campbell, Michael Millhollen, Lucy Fahler
Over the years, we have received a lot of questions about cytochrome P450 (CYP) 2C induction studies. In February of...
Which Hepatocytes Should I Use for What Studies?
- Test Systems & Methods
- March 25, 2022
- Dr. Chris Bohl, Michael Millhollen, Isis Smith
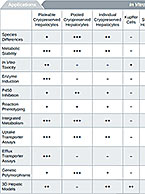
Primary hepatocytes are considered the gold standard for ADME/DMPK studies because they are the most representative in vitro test system. However, not all hepatocyte formats…
Meet the Scientist: Alex Wakefield
- Meet the Scientist
- February 1, 2022
- Michael Millhollen
In this month’s installment of our Meet the Scientist series we wanted to feature one of our team of talented...
Why Do Most Polled Researchers Run Red Blood Cell Partitioning Studies with Plasma Protein Binding?
- Drug Drug Interactions (DDI)
- January 25, 2022
- Dr. Steven McGreal, Michael Millhollen
Many compounds bind to or diffuse into red blood cells (RBCs), which can significantly impact clearance and cause inaccuracies in PK calculations...
Highlights from the 2021 Marbach Drug-Drug Interaction Workshop
- Drug Drug Interactions (DDI)
- January 11, 2022
- Darina Hynes
Reflecting on the virtual conferences in 2021, I would like to look back at some of the highlights from the...
2021 Service Expansions: Microsomal Protein Binding and Red Blood Cell Partitioning
- Updated Offerings
- January 3, 2022
- Michael Millhollen
Over the course of 2021, we expanded access to two important and related study offerings at our US laboratory headquarters....
Notes from the 2021 HRA Expert Panel Discussion “Are Standard Hepatocyte Models/Test Systems Still Good Enough?”
- Test Systems & Methods
- December 7, 2021
- Dr. Chris Bohl
MicroPhysiological Systems (MPS) is a general term used to describe miniaturized, in vitro test systems that couple microfluidics with advanced...
Meet the Scientists: Program Execution
- Meet the Scientist
- October 27, 2021
- Michael Millhollen
In this installment of our Meet the Scientist series, we asked several members from various areas of our Program Execution...
Interesting Topics at the 3rd SCI-RSC Symposium on Transporters in Drug Discovery and Development
- Drug Transporters
- September 22, 2021
- Andy Rhoades, Dr. Andrew G. Taylor, Michael Millhollen, Cody Hendren
A recap of some of the important and promising research that was presented at the Transporters in Drug Discovery and Development symposium...
Meet the Scientists: Analytical Services’ Chandra Kollu & Nadya Galeva
- Meet the Scientist
- September 21, 2021
- Michael Millhollen
This month we’re featuring two of the experts in our analytical services department: Senior Scientist Chandra Kollu and Mass Spectrometry...
ADME/PK & DDI Best Practices Industry Survey Results & Infographic
- Drug Metabolism
- September 12, 2021
- Michael Millhollen
With over 3500 respondents, only 4% of respondents said they had never experienced any repercussions from postponing these studies...
Guide to When & Why to Evaluate ADME/DMPK & Drug-Drug Interactions Available
- Consultancy
- September 12, 2021
- Michael Millhollen
In Safety first: Assessing drugs early can preclude regulatory and health issues, a new ebook developed by a collaboration with...
Meet the Scientist: Darina Hynes
- Meet the Scientist
- August 27, 2021
- Darina Hynes
Dr. Darina Hynes obtained her Ph.D. in Molecular Medicine from the Royal College of Surgeons in Ireland. She is currently a...
Why Switch to Hepatocyte Pellets?
- Test Systems & Methods
- June 30, 2021
- Michael Millhollen, Dr. Chris Bohl
You may have heard about the patented CryostaX® format of hepatocyte pellets, but do you know why XenoTech and countless other researchers have made the…
Four Ways CROs Drive Innovation for Improved In Vitro Drug Metabolism and Pharmacokinetics (DMPK) Studies
- Drug Metabolism
- May 17, 2021
- Darina Hynes, Madison Esely-Kohlman
Agility and focused growth allow drug developers to outsource expertise and benefit the industry at-large by fostering innovation. Panelists at the 2021 Annual DMDG Meeting…
DDI & Drug Repurposing Article featured in Drug Discovery World (DDW) Spring Edition 2021
- Drug Drug Interactions (DDI)
- April 29, 2021
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Repurposing (repositioning, re-profiling, or re-tasking) a drug potentially saves years of costly testing from going to waste and potentially providing a higher chance of success.…
Meet the Scientist: Stephanie Helmstetter
- Meet the Scientist
- February 20, 2021
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable product experts, Stephanie Helmstetter.
Interview with Outsourcing Pharma: Repurposing existing drugs accelerates discovery
- Consultancy
- January 18, 2021
- Madison Esely-Kohlman
“Exploring alternative uses for drugs tapped for other indications, can save considerable time and money in discovery, according to an...
Meet the Scientist: Lois Haupt
- Meet the Scientist
- November 6, 2020
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable Enzyme Inhibition experts, Lois Haupt.
Updated Look & Fresh Features: Welcome to Our Brand New Website!
- Updated Offerings
- September 18, 2020
- Madison Esely-Kohlman
We are thrilled to welcome you to the brand new XenoTech website! The reconstruction project is a massive overhaul of over 1,000 pages carefully recrafted…
Drug-Drug Interaction (DDI) Prediction Models Following In Vitro Studies in Preclinical Development
- Drug Drug Interactions (DDI)
- August 12, 2020
- Madison Esely-Kohlman, Dr. Pallavi Limaye, Andrea Wolff, Dr. Maciej Czerwinski
In preclinical development, a drug will be evaluated for potential to cause a drug-drug interaction (DDI) using in vitro experiments and then calculations that...
Toxicokinetic (TK) Analysis for Preclinical Drug Development
- In Vivo & Radiolabeling
- August 6, 2020
- Madison Esely-Kohlman, Jolanta Golec, Dr. Pallavi Limaye
The main goal of preclinical toxicokinetic (TK) studies is to establish a correlation between a candidate compound’s concentration or dose...
XenoTech Named Most Innovative Drug Development Solutions Provider – USA 2020
- Accomplishments
- August 3, 2020
- Michael Millhollen
The 2020 Technology Innovator Awards returns for its fifth year “to reward those talented and dedicated individuals and firms working...
Important DDI Considerations for Repurposing Drugs to Treat COVID-19
- Drug Drug Interactions (DDI)
- June 8, 2020
- Madison Esely-Kohlman
“Given the rapid spread of COVID-19 and its relatively high mortality, filling the gap for coronavirus-specific drugs is urgent. […]...
Missing Out on Annual ‘Comforting of the Soul’
- Our Team
- May 29, 2020
- Deja Coffin, Madison Esely-Kohlman, Jolanta Golec
As we begin this summer, many of us are grieving opportunities lost for parties and pool days with friends and...
What is DMPK and how does it fit into drug development?
- Drug Metabolism
- May 11, 2020
- Madison Esely-Kohlman
Drug metabolism and pharmacokinetics (DMPK) is a core discipline in drug development that considers the biotransformation of a drug compound...
Meet the Scientist: Pallavi Limaye
- Meet the Scientist
- April 28, 2020
- Madison Esely-Kohlman
We have welcomed a new consultant to the team! Dr. Pallavi Limaye now serves as a Director in the Scientific...
ADME and Drug-Drug Interactions for the Toxicologist
- Drug Drug Interactions (DDI)
- April 28, 2020
- Madison Esely-Kohlman
Highlights from the recent webinar presented by our newest expert consultant, Dr. Pallavi Limaye In her recent webinar (now available for...
New TMPRSS2-Expressing Cell Line for COVID-19 Research
- Updated Offerings
- April 23, 2020
- Madison Esely-Kohlman
We are offering a brand new cell line to assist researchers in the fight against COVID-19.The cell line (JCRB1819), developed...
What is ADME and how does it fit into drug development?
- Drug Metabolism
- April 10, 2020
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The main aim of drug development is to get a compound that has a therapeutic effect into the form of...
COVID-19 Updates
- Drug Drug Interactions (DDI)
- March 25, 2020
- Michael Millhollen
As a global Life Sciences and Healthcare company, XenoTech recognizes the seriousness of the Coronavirus Pandemic. We are constantly monitoring...
In Vitro Evaluation of Drug-Drug Interaction (DDI) Potential
- Enzyme Inhibition
- March 24, 2020
- Madison Esely-Kohlman, Zachary Mitts, Dr. Joanna Barbara
In its most recent in vitro drug interaction guidance update, the US Food and Drug Administration (FDA) emphasized harmony with the...
Four ways to optimize preclinical in vitro data to mitigate risk of late-stage clinical failure
- Consultancy
- March 9, 2020
- Madison Esely-Kohlman
1. Collect high-quality data to make informed, confident go/no go decisions for moving your drug candidate forward If you need...
How can in vitro and in vivo studies help me understand my drug’s clearance?
- Regulatory Guidance
- March 5, 2020
- Madison Esely-Kohlman, Dr. Joanna Barbara
Systemic clearance, denoting how much drug is cleared from a volume of blood per unit of time, is of critical...
KC Business Journal: KCK company helps Big Pharma save money at the molecular level
- Accomplishments
- February 27, 2020
- Lily Lieberman
Moving a new drug from lab to market is difficult, time-consuming and expensive for any size pharmaceutical or biotechnology company....
Meet the Scientist: Andrea Wolff
- Meet the Scientist
- February 20, 2020
- Michael Millhollen
At XenoTech, we pride ourselves on our 3S Principles – the values and standards that guide our businesses and shape...
Timing of In Vitro Studies: Early, Thorough ADME for Your Compound’s Success
- Regulatory Guidance
- February 13, 2020
- Madison Esely-Kohlman, Dr. Chris Bohl, Greg Loewen
Beyond the need for evidence that a drug works, Food and Drug Administration (FDA) and other regulatory bodies around the...
Meet the Scientist: Mark Horrigan
- Meet the Scientist
- January 27, 2020
- Michael Millhollen
Continuing our effort to put faces to the names of some of the scientists you interact with at XenoTech, we...
How Can I Make Sure My Data Meets Regulatory Expectations?
- Regulatory Guidance
- January 10, 2020
- Madison Esely-Kohlman, Greg Loewen
Regulatory authorities publish updated guidance documents that share their expectations for endpoints and test systems with drug developers, but sometimes it is difficult to...
What You Need to Know About Micro-Autoradiography (mARG) Distribution Studies
- In Vivo & Radiolabeling
- December 19, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo determination of drug localization in tissue can be uniquely informative to drug developers investigating distribution within the context of...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
- Regulatory Guidance
- November 25, 2019
- Madison Esely-Kohlman, Greg Loewen
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
DDSC In Vivo ADME Expertise Providing Cost Savings
- Updated Offerings
- November 19, 2019
- Scott Hickman
ADME studies are a vital part of the drug development process, and therefore, deciding where and how to perform these studies...
Mass Balance Studies: What You Need and Why You Need It
- In Vivo & Radiolabeling
- November 15, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo mass balance studies are an important element of nonclinical drug development, to inform first in-human (FIH) studies and to...
I Have My Data… Now What?
- Consultancy
- October 16, 2019
- Madison Esely-Kohlman
As a drug moves through the development pipeline, it undergoes a rigorous battery of safety assessments to prove it will...
The FDA has requested follow-up data… how do I fill in the gaps?
- Regulatory Guidance
- October 9, 2019
- Madison Esely-Kohlman
Because each new drug is unique in characteristics, such as chemical structure, mechanism of action, and physicochemical properties, there can...
Why You Need QWBA for Human Radiolabeled ADME Studies
- Regulatory Guidance
- October 4, 2019
- Satoshi Ito
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1. Quantitative whole body autoradiography (QWBA) is an in...
XenoTech Named Best In Vitro Drug CRO 2019
- Updated Offerings
- September 19, 2019
- Michael Millhollen
XenoTech has been named the Best In Vitro Drug CRO in the 2019 Healthcare & Pharmaceutical Awards. From the release:...
How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
XenoTech Named a 2019 Top-Performing Provider on Science Exchange
- Updated Offerings
- September 4, 2019
- Michael Millhollen
Science Exchange stated, “Discovery research programs at growing biotech companies are demanding—R&D leaders must balance the need to innovate with...
Cypex Adds Recombinant UGTs
- Updated Offerings
- September 3, 2019
- Madison Esely-Kohlman
Recombinant enzymes represent a useful test system for studies requiring extra high levels of catalytic activity. Due to high customer demand, Cypex has...
Consultancy Expansion
- Consultancy
- August 20, 2019
- Madison Esely-Kohlman
We are proud to house some of the brightest minds in this field who have seen “the weird stuff” when...
Can CYP3A4 Induction Predict P-glycoprotein Induction in DDI Studies?
- Enzyme Induction
- August 20, 2019
- Shanté Jackson, Madison Esely-Kohlman
Generally, a drug’s effects on enzyme and transporter activity are examined independently in a drug development program, but what if...
Reduced Pricing for In Vitro Transporter Studies
- Updated Offerings
- August 20, 2019
- Madison Esely-Kohlman
We’re pleased to announce that our scientists’ ongoing dedication to enhancing our processes and discovering new efficiencies without sacrificing quality...
XenoTech Named Best for Drug Candidate Evaluations 2019, Leading Providers of Pharmaceuticals Safety Testing
- Updated Offerings
- August 12, 2019
- Michael Millhollen
XenoTech has been named the Best for Drug Candidate Evaluations 2019 as well as in the Leading Providers of Pharmaceuticals...
In Vivo ADME: What You Need and Why You Need It
- In Vivo & Radiolabeling
- July 22, 2019
- Madison Esely-Kohlman, Satoshi Ito
When putting together a data package for regulatory approval by the FDA, EMA, or PMDA, there is a lot to...
The Story of Us
- Updated Offerings
- July 1, 2019
- Madison Esely-Kohlman, Satoshi Ito
This year at XenoTech we celebrate our 25th birthday, and our partner in Japan turns 64! Looking back over our history,...
Transporters of Emerging Importance in Drug Development: Beyond the Guidance Documents
- Regulatory Guidance
- June 25, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Dr. Ogilvie’s presentation discusses critical literature and case studies which have been published following the FDA’s 2017 guidance revision, and covered...
Cell Handling & Media Selection for Best Results in Hepatocyte Assays
- Test Systems & Methods
- June 19, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Getting reliable, reproducible results from studies using hepatocytes as a model system for drug metabolism is in part dependent on how well...
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
- Enzyme Induction
- May 13, 2019
- Madison Esely-Kohlman, Dr. Joanna Barbara, Rebecca Campbell
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
Official PMDA English Translation
- Regulatory Guidance
- May 7, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The Japanese regulatory agent PMDA (Pharmaceuticals and Medical Devices Agency) recently published an official English translation of their final Drug Interaction Guideline...
Quality Control is What Makes the Comprehensive Collection of Cell Lines from JCRB Among the Highest Regarded and Most Widely Distributed in the World
- Test Systems & Methods
- April 23, 2019
- Madison Esely-Kohlman, Dr. Arihiro Kohara, Kimiho Yamada
About JCRB cell bank In addition to focus on preclinical ADME in vitro testing services and complementary products, XenoTech offers to our clients...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
- Test Systems & Methods
- April 15, 2019
- Madison Esely-Kohlman, Andrea Wolff, Dr. Joanna Barbara
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
New Liver Tissue Microarrays Available – What Would Benefit Your Research?
- Disease-Specific Resources
- April 2, 2019
- Madison Esely-Kohlman, Dr. Maciej Czerwinski
As technology evolves to better meet needs in exploration of drug metabolism pathways and disease mechanisms, familiarization with appropriate and...
Disease-State Test Systems
- Test Systems & Methods
- January 13, 2019
- Michael Millhollen, Dr. Chris Bohl
XenoTech has been working with and supplying reagents for the pre-clinical ADME field for well over two decades. Years of...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
5 Keys to Set Your Contract Research Organization (CRO) Collaboration Up for Success
- Updated Offerings
- January 7, 2019
- Michael Millhollen, Kelsey Acree
Over the past 25 years of performing ADME/DMPK/DDI contract research, we’ve learned some key strategies to maximize the efficiency and effectiveness...
Holiday CSR Activities
- Updated Offerings
- December 26, 2018
- Michael Millhollen, Deja Coffin
XenoTech understands that a better future starts with our actions today, and encourages its global family to remain focused on...
FDA Guidance: Many In Vitro DDI Evaluations Should Precede FIH Studies
- Regulatory Guidance
- December 6, 2018
- Dr. Brian Ogilvie, Andrea Wolff
In October 2017, the FDA released its much-anticipated draft guidance documents for drug-drug interaction (DDI) studies, which was finalized in...
Coverage from the October 2018 PBSS DDI Workshop
- Drug Transporters
- November 5, 2018
- Greg Loewen, Michael Millhollen
The Pharmaceutical and BioScience Society (PBSS) hosted the workshop “Drug-Drug Interactions: Update on Risk Assessment, Clinical Evaluation and Regulatory Requirements” on October...
October Presentations on In Vitro Effects of Biologics on CYP Enzymes and Regulatory DDI Guidances
- Regulatory Guidance
- October 26, 2018
- Michael Millhollen
On Oct. 15th at the Peptide ADME Discussion Group Workshop in Gothenburg, Sweden, Dr. Brian Ogilvie presented on In vitro Direct and Cytokine-Mediated...
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
New Recommendation: Evaluate NMEs for OCT1 Transporter-Mediated DDI Potential
- Drug Transporters
- October 1, 2018
- Dr. Brian Ogilvie, Andrea Wolff
Brian Ogilvie, Ph.D., Vice President of Scientific Consulting at XenoTech, presented a case study on the importance of the hepatic OCT1...
Cypex Expands Portfolio of Recombinant Enzymes
- Non-CYP Enzymes
- September 18, 2018
- Scott Hickman
As a distributor for Cypex in the US and Canada, XenoTech is proud to offer this portfolio of products based on patented...
New Rodent, Monkey CryostaX Hepatocytes Featured in Industry News
- Test Systems & Methods
- September 13, 2018
- Michael Millhollen
Following XenoTech’s announcement XenoTech Adds Monkey, Rodent Hepatocytes to Patented CryostaX Product Line, Anticipates the End of Traditionally Cryopreserved Hepatocytes, various...
Challenges & Solutions in Today’s In Vitro Transporter Research
- Drug Transporters
- August 30, 2018
- Michael Millhollen, Dr. Joanna Barbara
Transporters are membrane-bound proteins that govern the passage of drugs into and out of cells. These gatekeeper proteins can be...
XenoTech Named Best Global CRO 2018; Recognised Leaders in Pharmaceutical Safety Testing 2018
- Updated Offerings
- August 24, 2018
- Michael Millhollen
XenoTech has been named the Best Global CRO and was recognized for leadership in Pharmaceutical Safety Testing in the 2018...
Response to FDA Framework for Assessment of Drug-Drug Interactions for Therapeutic Proteins RFI and Comments
- Regulatory Guidance
- August 6, 2018
- Michael Millhollen
In July, Drs. Maciej Czerwinski, Director of Consulting, and Brian Ogilvie, Vice President of Scientific Consulting, submitted XenoTech’s comments in response to the Food...
IQ Induction Working Group Publishes Recommendations for Data Interpretation of In Vitro Induction – Focus on CYP3A4 mRNA
- Enzyme Induction
- July 13, 2018
- Scott Hickman, Dr. Brian Ogilvie
XenoTech is proud to have made contributions to the paper entitled, “Considerations from the IQ Induction Working Group in Response...
New Guidance on Bioanalytical Services
- Regulatory Guidance
- July 6, 2018
- Scott Hickman, Seema Muranjan
XenoTech offers bioanalytical services to support both internal groups and external clients. We have validated LC-MS/MS methods for human CYP markers, UDP...
No-Cost, On-Demand Primary Human Hepatocyte Pooling Using CryostaX Pellets
- Test Systems & Methods
- June 15, 2018
- Michael Millhollen, Dr. Chris Bohl
CryostaX® hepatocytes are created using a patented process that produces unique single-donor cell pellets. This format allows scientists to easily pool primary...
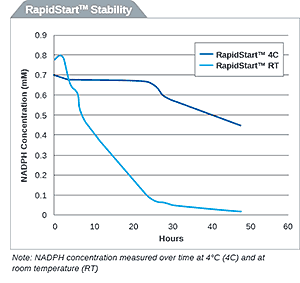
NADPH RapidStart Regeneration System for Extended Metabolism
- Test Systems & Methods
- May 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. NADPH is a critical cofactor...
AAPS-FDA Drug Transporters
- Regulatory Guidance
- May 30, 2018
- Greg Loewen, Michael Millhollen
The American Association of Pharmaceutical Scientists (AAPS) and U.S. Food and Drug Administration (FDA) co-sponsored the workshop “Drug Transporters in ADME:...
In Vitro DDI Regulatory Guidance Reference Poster
- Regulatory Guidance
- May 29, 2018
- Michael Millhollen
At the 2018 Marbach Castle Drug-Drug Interaction Workshop in Germany, Dr. Brian Ogilvie, Vice President of Scientific Consulting, presented a comparison...
Maximize Metabolic Activities by Limiting Hepatocyte Cryoinjury
- Drug Metabolism
- May 1, 2018
- Michael Millhollen, Dr. Chris Bohl
Cryopreservation and thawing of primary hepatocytes causes inherent damage to the cells. Traditional pooling methods require cells to undergo two separate cryopreservation...
Assess Catabolic Stability of Biologics & ADCs with Lysosomes – Characterized Test Systems
- Test Systems & Methods
- April 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. Following up on my series...
No More Water Baths! Simplify Hepatocyte Thaws and Eliminate Contamination
- Test Systems & Methods
- April 3, 2018
- Michael Millhollen, Dr. Chris Bohl
XenoTech has patented technology that eliminates the need for the water baths that are traditionally required to thaw cryopreserved hepatocytes. By removing water...
XenoTech Adds US Support for MATE1 and MATE2-K Drug Transporter Studies
- Drug Transporters
- April 2, 2018
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best In Vitro Drug Metabolism Studies Provider 2018
- Accomplishments
- March 27, 2018
- Michael Millhollen
Following our nomination in late 2017, XenoTech has been named the Best In Vitro Drug Metabolism Studies Provider in the...
Choosing a Relevant Small Animal Model for Pharmacokinetic or Toxicity Studies
- Test Systems & Methods
- March 1, 2018
- Dr. Chris Bohl
Choosing the most suitable small animal model is crucial for pharmacokinetic and/or toxicology studies in order to get usable, relevant data. Due to potential...
Recombinant CYP Bactosomes: Versatile Formats for Many ADME Applications
- Test Systems & Methods
- February 25, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase recombinant CYPs in the EU, please visit tebu-bio’s website. For North American orders, please follow...
Insects and Cattle and Sheep, Oh My!
- Test Systems & Methods
- February 15, 2018
- Michael Millhollen, Aaron Hilgedick
Over the past two decades, we’ve received a lot of interesting requests for custom tissue preparations here at XenoTech. “We’ve...
XenoTech Hiring and Growing to Meet Customer Demand
- Updated Offerings
- January 8, 2018
- Michael Millhollen
As announced earlier this month, XenoTech has hired a record number of new staff over the past year in order to...
What To Do with Microsome Stable Low Turnover Compounds
- Drug Metabolism
- December 7, 2017
- Dr. Chris Bohl
Compounds that exhibit high metabolic stability in hepatocytes and subcellular fractions (S9, microsomes and cytosol) can be a challenge for ADME scientists. These in...
XenoTech Featured in Fall Issue of NewsWave
- Accomplishments
- November 15, 2017
- Michael Millhollen
Leader in pharmaceutical research and scientific collaboration Originally printed in the Fall 2017 Issue of NewsWave. XenoTech, LLC, which is...
Initial Impressions of New Draft FDA DDI Guidance Documents from XenoTech
- Regulatory Guidance
- October 26, 2017
- Michael Millhollen, Dr. Brian Ogilvie
Updated Nov. 6th, 2017 The FDA has released its long-awaited new draft guidance for industry on drug-drug interaction (DDI) studies....
HepatoSure Hepatocytes: The Largest Donor Pool For Your DMPK Applications
- Drug Metabolism
- October 18, 2017
- Isabelle Nobiron
Pooled human hepatocytes can complement a selection of hepatocytes from various species for metabolite profiling, species comparison and evaluation of potential human-specific metabolites. HepatoSure® is the...
Further Research on the Drug-Drug Interaction Between Gemfibrozil and Repaglinide Presented
- Drug Drug Interactions (DDI)
- September 27, 2017
- Michael Millhollen
The clinically-relevant drug-drug interaction (DDI) between the dyslipidemia drug gemfibrozil and the antidiabetic repaglinide is well-documented throughout the literature. In...
Cold Storage Solution Linked to Lower AO, XO Activity
- Drug Metabolism
- August 22, 2017
- Michael Millhollen
At the time of organ recovery, human livers that have been donated for research are flushed with an ice-cold perfusion...
Considerations In Response to Drug-Drug Interaction Guidances
- Regulatory Guidance
- August 17, 2017
- Michael Millhollen
Check out the recent IQ consortium publication: Considerations from the IQ Induction Working Group in Response to Drug-Drug Interaction Guidances from...
URI Drug Transporters Workshop Presentations
- Drug Transporters
- August 11, 2017
- Michael Millhollen
The University of Rhode Island College of Pharmacy’s 5th Annual Transporters in Drug Discovery and Development: Driving Knowledge from Laboratory...
New Division Directors for Core Services and Logistics
- Updated Offerings
- August 1, 2017
- Michael Millhollen
Following the promotion of Dr. Joanna Barbara, PhD, to Vice President of Scientific Operations, Dr. Etsuko Usuki, PhD, has been promoted...
XenoTech’s Analytical Services Department Adds Instrumentation
- Updated Offerings
- July 17, 2017
- Michael Millhollen
XenoTech’s Analytical Services lab provides researchers with custom method development and method validation, dose solution analysis, in vitro and in vivo metabolite profiling studies, small-molecule non-GLP bioanalysis and...
Considerations When Studying Esterase Activities in the Intestine
- Drug Metabolism
- July 12, 2017
- Dr. Chris Bohl, Michael Millhollen
The gastrointestinal wall is a significant site of first-pass metabolism for oral drugs, which is commonly associated with CYP450 and...
UGT Activities, Concomitant Drugs, and DDI
- Enzyme Induction
- June 20, 2017
- Michael Millhollen
If you have concerns about how your compound may affect UDP-glucuronosyltransferase (UGT) induction and/or inhibition when combined with other therapeutic...
New range of Pig P450s Bactosomes
- Updated Offerings
- June 19, 2017
- Isabelle Nobiron
If you are looking for enzymatic in vitro tools for drug metabolism, reaction phenotyping or metabolite generation using a pig model, you may find these newly...
New Liver Disease Resource and Other Research Biobank News
- Updated Offerings
- May 26, 2017
- Michael Millhollen, Dr. Maciej Czerwinski
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, which affects one out of four people worldwide, such as...
Big Hepatocyte News!
- Test Systems & Methods
- May 10, 2017
- Michael Millhollen
As you may have heard, XenoTech was issued U.S. Patent No. 9,642,355 for the “CRYOPRESERVATION OF CELLS AND SUBCELLULAR FRACTIONS” for its CryostaX® hepatocytes. The...
New VP of Scientific Operations and VP of Scientific Consulting
- Consultancy
- May 4, 2017
- Michael Millhollen
XenoTech has appointed Joanna Barbara, Ph.D., as Vice President of Scientific Operations and Brian Ogilvie, Ph.D., as Vice President of Scientific...
New Lysosome Catabolism Protocol and Tech Tips
- Biologics & Oligonucleotides
- April 17, 2017
- Dr. Chris Bohl
A new guide outlining our IgG Catabolism Protocol as well as Lysosome and Tritosome Technical Tips is now available for those evaluating lysosomal stability...
XenoTech Adds New Drug Transporters to Portfolio
- Drug Transporters
- April 1, 2017
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best for Pharmaceutical Safety Testing 2017
- Accomplishments
- March 2, 2017
- Michael Millhollen
After our nomination in late 2016, XenoTech has been named the Best for Pharmaceutical Safety Testing in the 2017 Biotechnology...
XenoTech Adds New Dermal Subcellular Fraction Test Systems
- Updated Offerings
- February 1, 2017
- Michael Millhollen
XenoTech is adding human and minipig to the company’s list of species with dermal subcellular fractions available as standard test systems for the development...
Pharmaceutical Outsourcing Magazine Interview Nov-Dec 2016
- Updated Offerings
- November 29, 2016
- Michael Millhollen
For their November/December 2016 issue, Pharmaceutical Outsourcing Magazine interviewed Chris Bohl, PhD, Global Technical Support Manager for XenoTech’s Products Division, on the...
XenoTech Standardizes Lysosomal Test Systems for Biopharmaceutical Development
- Test Systems & Methods
- October 5, 2016
- Dr. Chris Bohl
Before releasing these test systems, there was not a high quality, specific, relevant, easy and ready to use, in vitro test system to...
XenoTech Joins Fight Against Liver Disease
- Disease-Specific Resources
- September 16, 2016
- Dr. Maciej Czerwinski, Michael Millhollen
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, such as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis...
XenoTech Featured on Labiotech.EU
- Enzyme Inhibition
- July 5, 2016
- Michael Millhollen
Understand which transporters are involved in a drug’s absorption, distribution & excretion Originally posted on Labiotech.EU How can you build the...
XenoTech Scientists Publish Paper, Present Research Evaluating Ketoconazole and its Alternative Clinical CYP3A4-5 Inhibitors as Inhibitors of Drug Transporters
- Enzyme Inhibition
- April 25, 2016
- Michael Millhollen
XenoTech scientists published a paper in Drug Metabolism and Disposition evaluating Ketoconazole and its alternative clinical CYP3A4-5 inhibitors as inhibitors of drug...
XenoTech Fulfills Largest Order To Date
- Accomplishments
- February 1, 2016
- Michael Millhollen
XenoTech recently fulfilled its largest order of human liver subcellular fractions to date. A large pharmaceutical customer ordered over 30,000...
XenoTech Hosts Bring Your Kids to Work Day
- Our Team
- May 4, 2015
- Matthew Beck, Michael Millhollen
On Thursday, April 23rd, XenoTech participated in “bring your child to work day”. At XenoTech, we are passionate about what...
Navigating the Transporter Wave of 2013
- Regulatory Guidance
- August 28, 2013
- Greg Loewen
The International Transporter Consortium (ITC) issued 7 papers in the July 2013 issue of Clinical Pharmacology & Therapeutics. These 7...
FAQ: Using Plateable Hepatocytes in In Vitro Assays
- Test Systems & Methods
- December 13, 2022
- Halee McElhaney, Lucy Fahler
Plateable hepatocytes are often used for induction studies and to research metabolism, biochemistry, virology, host/pathogen interactions, and cell biology. Here,...
Ask Me Anything About In Vitro ADME, Drug-Drug Interaction Approaches and Strategies
- ADME 101™
- December 1, 2022
- Dr. Joanna Barbara
Last month, Dr. Joanna Barbara joined AAPS for an online Q&A in their ongoing “Ask the Experts” series. As regulatory...
Are in vitro drug metabolism and drug-drug interaction studies critical for an IND?
- ADME 101™
- November 1, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith, Amara Millhollen
A Guide to What, Why & When to Conduct ADME Studies When drug developers are preparing their IND, drug metabolism...
4 Ways De-Risking Maximizes Compound Value
- ADME 101™
- August 31, 2022
- Michael Millhollen
Since most new drugs fail because of ADME/Tox, you can add considerable value to your compound by conducting early in...
New FDA Draft Guidance “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics” – XenoTech’s perspective on in vitro DDI testing
- Regulatory Guidance
- July 11, 2022
- Dr. Maciej Czerwinski, Dr. Pallavi Limaye, Dr. Brian Ogilvie
The FDA has released a new draft guidance for industry titled “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics.”...
Spotlight on Efflux and Uptake Drug Transporters in In Vitro Drug-Drug Interaction Studies
- Drug Transporters
- June 23, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith
What are drug transporters? Drug transporters are membrane-bound proteins that assist in the movement of drugs into or out of...
When, Why and How to Conduct CYP2C Induction Studies
- Enzyme Induction
- April 8, 2022
- Dr. Andrew G. Taylor, Rebecca Campbell, Michael Millhollen, Lucy Fahler
Over the years, we have received a lot of questions about cytochrome P450 (CYP) 2C induction studies. In February of...
Which Hepatocytes Should I Use for What Studies?
- Test Systems & Methods
- March 25, 2022
- Dr. Chris Bohl, Michael Millhollen, Isis Smith
Primary hepatocytes are considered the gold standard for ADME/DMPK studies because they are the most representative in vitro test system. However, not all hepatocyte formats…
Meet the Scientist: Alex Wakefield
- Meet the Scientist
- February 1, 2022
- Michael Millhollen
In this month’s installment of our Meet the Scientist series we wanted to feature one of our team of talented...
Why Do Most Polled Researchers Run Red Blood Cell Partitioning Studies with Plasma Protein Binding?
- Drug Drug Interactions (DDI)
- January 25, 2022
- Dr. Steven McGreal, Michael Millhollen
Many compounds bind to or diffuse into red blood cells (RBCs), which can significantly impact clearance and cause inaccuracies in PK calculations...
Highlights from the 2021 Marbach Drug-Drug Interaction Workshop
- Drug Drug Interactions (DDI)
- January 11, 2022
- Darina Hynes
Reflecting on the virtual conferences in 2021, I would like to look back at some of the highlights from the...
2021 Service Expansions: Microsomal Protein Binding and Red Blood Cell Partitioning
- Updated Offerings
- January 3, 2022
- Michael Millhollen
Over the course of 2021, we expanded access to two important and related study offerings at our US laboratory headquarters....
Notes from the 2021 HRA Expert Panel Discussion “Are Standard Hepatocyte Models/Test Systems Still Good Enough?”
- Test Systems & Methods
- December 7, 2021
- Dr. Chris Bohl
MicroPhysiological Systems (MPS) is a general term used to describe miniaturized, in vitro test systems that couple microfluidics with advanced...
Meet the Scientists: Program Execution
- Meet the Scientist
- October 27, 2021
- Michael Millhollen
In this installment of our Meet the Scientist series, we asked several members from various areas of our Program Execution...
Interesting Topics at the 3rd SCI-RSC Symposium on Transporters in Drug Discovery and Development
- Drug Transporters
- September 22, 2021
- Andy Rhoades, Dr. Andrew G. Taylor, Michael Millhollen, Cody Hendren
A recap of some of the important and promising research that was presented at the Transporters in Drug Discovery and Development symposium...
Meet the Scientists: Analytical Services’ Chandra Kollu & Nadya Galeva
- Meet the Scientist
- September 21, 2021
- Michael Millhollen
This month we’re featuring two of the experts in our analytical services department: Senior Scientist Chandra Kollu and Mass Spectrometry...
ADME/PK & DDI Best Practices Industry Survey Results & Infographic
- Drug Metabolism
- September 12, 2021
- Michael Millhollen
With over 3500 respondents, only 4% of respondents said they had never experienced any repercussions from postponing these studies...
Guide to When & Why to Evaluate ADME/DMPK & Drug-Drug Interactions Available
- Consultancy
- September 12, 2021
- Michael Millhollen
In Safety first: Assessing drugs early can preclude regulatory and health issues, a new ebook developed by a collaboration with...
Meet the Scientist: Darina Hynes
- Meet the Scientist
- August 27, 2021
- Darina Hynes
Dr. Darina Hynes obtained her Ph.D. in Molecular Medicine from the Royal College of Surgeons in Ireland. She is currently a...
Why Switch to Hepatocyte Pellets?
- Test Systems & Methods
- June 30, 2021
- Michael Millhollen, Dr. Chris Bohl
You may have heard about the patented CryostaX® format of hepatocyte pellets, but do you know why XenoTech and countless other researchers have made the…
Four Ways CROs Drive Innovation for Improved In Vitro Drug Metabolism and Pharmacokinetics (DMPK) Studies
- Drug Metabolism
- May 17, 2021
- Darina Hynes, Madison Esely-Kohlman
Agility and focused growth allow drug developers to outsource expertise and benefit the industry at-large by fostering innovation. Panelists at the 2021 Annual DMDG Meeting…
DDI & Drug Repurposing Article featured in Drug Discovery World (DDW) Spring Edition 2021
- Drug Drug Interactions (DDI)
- April 29, 2021
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Repurposing (repositioning, re-profiling, or re-tasking) a drug potentially saves years of costly testing from going to waste and potentially providing a higher chance of success.…
Meet the Scientist: Stephanie Helmstetter
- Meet the Scientist
- February 20, 2021
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable product experts, Stephanie Helmstetter.
Interview with Outsourcing Pharma: Repurposing existing drugs accelerates discovery
- Consultancy
- January 18, 2021
- Madison Esely-Kohlman
“Exploring alternative uses for drugs tapped for other indications, can save considerable time and money in discovery, according to an...
Meet the Scientist: Lois Haupt
- Meet the Scientist
- November 6, 2020
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable Enzyme Inhibition experts, Lois Haupt.
Updated Look & Fresh Features: Welcome to Our Brand New Website!
- Updated Offerings
- September 18, 2020
- Madison Esely-Kohlman
We are thrilled to welcome you to the brand new XenoTech website! The reconstruction project is a massive overhaul of over 1,000 pages carefully recrafted…
Drug-Drug Interaction (DDI) Prediction Models Following In Vitro Studies in Preclinical Development
- Drug Drug Interactions (DDI)
- August 12, 2020
- Madison Esely-Kohlman, Dr. Pallavi Limaye, Andrea Wolff, Dr. Maciej Czerwinski
In preclinical development, a drug will be evaluated for potential to cause a drug-drug interaction (DDI) using in vitro experiments and then calculations that...
Toxicokinetic (TK) Analysis for Preclinical Drug Development
- In Vivo & Radiolabeling
- August 6, 2020
- Madison Esely-Kohlman, Jolanta Golec, Dr. Pallavi Limaye
The main goal of preclinical toxicokinetic (TK) studies is to establish a correlation between a candidate compound’s concentration or dose...
XenoTech Named Most Innovative Drug Development Solutions Provider – USA 2020
- Accomplishments
- August 3, 2020
- Michael Millhollen
The 2020 Technology Innovator Awards returns for its fifth year “to reward those talented and dedicated individuals and firms working...
Important DDI Considerations for Repurposing Drugs to Treat COVID-19
- Drug Drug Interactions (DDI)
- June 8, 2020
- Madison Esely-Kohlman
“Given the rapid spread of COVID-19 and its relatively high mortality, filling the gap for coronavirus-specific drugs is urgent. […]...
Missing Out on Annual ‘Comforting of the Soul’
- Our Team
- May 29, 2020
- Deja Coffin, Madison Esely-Kohlman, Jolanta Golec
As we begin this summer, many of us are grieving opportunities lost for parties and pool days with friends and...
What is DMPK and how does it fit into drug development?
- Drug Metabolism
- May 11, 2020
- Madison Esely-Kohlman
Drug metabolism and pharmacokinetics (DMPK) is a core discipline in drug development that considers the biotransformation of a drug compound...
Meet the Scientist: Pallavi Limaye
- Meet the Scientist
- April 28, 2020
- Madison Esely-Kohlman
We have welcomed a new consultant to the team! Dr. Pallavi Limaye now serves as a Director in the Scientific...
ADME and Drug-Drug Interactions for the Toxicologist
- Drug Drug Interactions (DDI)
- April 28, 2020
- Madison Esely-Kohlman
Highlights from the recent webinar presented by our newest expert consultant, Dr. Pallavi Limaye In her recent webinar (now available for...
New TMPRSS2-Expressing Cell Line for COVID-19 Research
- Updated Offerings
- April 23, 2020
- Madison Esely-Kohlman
We are offering a brand new cell line to assist researchers in the fight against COVID-19.The cell line (JCRB1819), developed...
What is ADME and how does it fit into drug development?
- Drug Metabolism
- April 10, 2020
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The main aim of drug development is to get a compound that has a therapeutic effect into the form of...
COVID-19 Updates
- Drug Drug Interactions (DDI)
- March 25, 2020
- Michael Millhollen
As a global Life Sciences and Healthcare company, XenoTech recognizes the seriousness of the Coronavirus Pandemic. We are constantly monitoring...
In Vitro Evaluation of Drug-Drug Interaction (DDI) Potential
- Enzyme Inhibition
- March 24, 2020
- Madison Esely-Kohlman, Zachary Mitts, Dr. Joanna Barbara
In its most recent in vitro drug interaction guidance update, the US Food and Drug Administration (FDA) emphasized harmony with the...
Four ways to optimize preclinical in vitro data to mitigate risk of late-stage clinical failure
- Consultancy
- March 9, 2020
- Madison Esely-Kohlman
1. Collect high-quality data to make informed, confident go/no go decisions for moving your drug candidate forward If you need...
How can in vitro and in vivo studies help me understand my drug’s clearance?
- Regulatory Guidance
- March 5, 2020
- Madison Esely-Kohlman, Dr. Joanna Barbara
Systemic clearance, denoting how much drug is cleared from a volume of blood per unit of time, is of critical...
KC Business Journal: KCK company helps Big Pharma save money at the molecular level
- Accomplishments
- February 27, 2020
- Lily Lieberman
Moving a new drug from lab to market is difficult, time-consuming and expensive for any size pharmaceutical or biotechnology company....
Meet the Scientist: Andrea Wolff
- Meet the Scientist
- February 20, 2020
- Michael Millhollen
At XenoTech, we pride ourselves on our 3S Principles – the values and standards that guide our businesses and shape...
Timing of In Vitro Studies: Early, Thorough ADME for Your Compound’s Success
- Regulatory Guidance
- February 13, 2020
- Madison Esely-Kohlman, Dr. Chris Bohl, Greg Loewen
Beyond the need for evidence that a drug works, Food and Drug Administration (FDA) and other regulatory bodies around the...
Meet the Scientist: Mark Horrigan
- Meet the Scientist
- January 27, 2020
- Michael Millhollen
Continuing our effort to put faces to the names of some of the scientists you interact with at XenoTech, we...
How Can I Make Sure My Data Meets Regulatory Expectations?
- Regulatory Guidance
- January 10, 2020
- Madison Esely-Kohlman, Greg Loewen
Regulatory authorities publish updated guidance documents that share their expectations for endpoints and test systems with drug developers, but sometimes it is difficult to...
What You Need to Know About Micro-Autoradiography (mARG) Distribution Studies
- In Vivo & Radiolabeling
- December 19, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo determination of drug localization in tissue can be uniquely informative to drug developers investigating distribution within the context of...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
- Regulatory Guidance
- November 25, 2019
- Madison Esely-Kohlman, Greg Loewen
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
DDSC In Vivo ADME Expertise Providing Cost Savings
- Updated Offerings
- November 19, 2019
- Scott Hickman
ADME studies are a vital part of the drug development process, and therefore, deciding where and how to perform these studies...
Mass Balance Studies: What You Need and Why You Need It
- In Vivo & Radiolabeling
- November 15, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo mass balance studies are an important element of nonclinical drug development, to inform first in-human (FIH) studies and to...
I Have My Data… Now What?
- Consultancy
- October 16, 2019
- Madison Esely-Kohlman
As a drug moves through the development pipeline, it undergoes a rigorous battery of safety assessments to prove it will...
The FDA has requested follow-up data… how do I fill in the gaps?
- Regulatory Guidance
- October 9, 2019
- Madison Esely-Kohlman
Because each new drug is unique in characteristics, such as chemical structure, mechanism of action, and physicochemical properties, there can...
Why You Need QWBA for Human Radiolabeled ADME Studies
- Regulatory Guidance
- October 4, 2019
- Satoshi Ito
A quantitative whole body autoradiography (QWBA) study provides data required for Human Radiolabeled ADME Studies1. Quantitative whole body autoradiography (QWBA) is an in...
XenoTech Named Best In Vitro Drug CRO 2019
- Updated Offerings
- September 19, 2019
- Michael Millhollen
XenoTech has been named the Best In Vitro Drug CRO in the 2019 Healthcare & Pharmaceutical Awards. From the release:...
How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
XenoTech Named a 2019 Top-Performing Provider on Science Exchange
- Updated Offerings
- September 4, 2019
- Michael Millhollen
Science Exchange stated, “Discovery research programs at growing biotech companies are demanding—R&D leaders must balance the need to innovate with...
Cypex Adds Recombinant UGTs
- Updated Offerings
- September 3, 2019
- Madison Esely-Kohlman
Recombinant enzymes represent a useful test system for studies requiring extra high levels of catalytic activity. Due to high customer demand, Cypex has...
Consultancy Expansion
- Consultancy
- August 20, 2019
- Madison Esely-Kohlman
We are proud to house some of the brightest minds in this field who have seen “the weird stuff” when...
Can CYP3A4 Induction Predict P-glycoprotein Induction in DDI Studies?
- Enzyme Induction
- August 20, 2019
- Shanté Jackson, Madison Esely-Kohlman
Generally, a drug’s effects on enzyme and transporter activity are examined independently in a drug development program, but what if...
Reduced Pricing for In Vitro Transporter Studies
- Updated Offerings
- August 20, 2019
- Madison Esely-Kohlman
We’re pleased to announce that our scientists’ ongoing dedication to enhancing our processes and discovering new efficiencies without sacrificing quality...
XenoTech Named Best for Drug Candidate Evaluations 2019, Leading Providers of Pharmaceuticals Safety Testing
- Updated Offerings
- August 12, 2019
- Michael Millhollen
XenoTech has been named the Best for Drug Candidate Evaluations 2019 as well as in the Leading Providers of Pharmaceuticals...
In Vivo ADME: What You Need and Why You Need It
- In Vivo & Radiolabeling
- July 22, 2019
- Madison Esely-Kohlman, Satoshi Ito
When putting together a data package for regulatory approval by the FDA, EMA, or PMDA, there is a lot to...
The Story of Us
- Updated Offerings
- July 1, 2019
- Madison Esely-Kohlman, Satoshi Ito
This year at XenoTech we celebrate our 25th birthday, and our partner in Japan turns 64! Looking back over our history,...
Transporters of Emerging Importance in Drug Development: Beyond the Guidance Documents
- Regulatory Guidance
- June 25, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Dr. Ogilvie’s presentation discusses critical literature and case studies which have been published following the FDA’s 2017 guidance revision, and covered...
Cell Handling & Media Selection for Best Results in Hepatocyte Assays
- Test Systems & Methods
- June 19, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Getting reliable, reproducible results from studies using hepatocytes as a model system for drug metabolism is in part dependent on how well...
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
- Enzyme Induction
- May 13, 2019
- Madison Esely-Kohlman, Dr. Joanna Barbara, Rebecca Campbell
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
Official PMDA English Translation
- Regulatory Guidance
- May 7, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The Japanese regulatory agent PMDA (Pharmaceuticals and Medical Devices Agency) recently published an official English translation of their final Drug Interaction Guideline...
Quality Control is What Makes the Comprehensive Collection of Cell Lines from JCRB Among the Highest Regarded and Most Widely Distributed in the World
- Test Systems & Methods
- April 23, 2019
- Madison Esely-Kohlman, Dr. Arihiro Kohara, Kimiho Yamada
About JCRB cell bank In addition to focus on preclinical ADME in vitro testing services and complementary products, XenoTech offers to our clients...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
- Test Systems & Methods
- April 15, 2019
- Madison Esely-Kohlman, Andrea Wolff, Dr. Joanna Barbara
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
New Liver Tissue Microarrays Available – What Would Benefit Your Research?
- Disease-Specific Resources
- April 2, 2019
- Madison Esely-Kohlman, Dr. Maciej Czerwinski
As technology evolves to better meet needs in exploration of drug metabolism pathways and disease mechanisms, familiarization with appropriate and...
Disease-State Test Systems
- Test Systems & Methods
- January 13, 2019
- Michael Millhollen, Dr. Chris Bohl
XenoTech has been working with and supplying reagents for the pre-clinical ADME field for well over two decades. Years of...
Important Considerations for the Conduct of In Vitro Drug Transporter Assays
- Drug Transporters
- January 11, 2019
- Greg Loewen, Andrea Wolff, Michael Millhollen
Not only can drug transporters affect the absorption and excretion of drugs, they can be involved in pharmacokinetic-based drug-drug interactions...
5 Keys to Set Your Contract Research Organization (CRO) Collaboration Up for Success
- Updated Offerings
- January 7, 2019
- Michael Millhollen, Kelsey Acree
Over the past 25 years of performing ADME/DMPK/DDI contract research, we’ve learned some key strategies to maximize the efficiency and effectiveness...
Holiday CSR Activities
- Updated Offerings
- December 26, 2018
- Michael Millhollen, Deja Coffin
XenoTech understands that a better future starts with our actions today, and encourages its global family to remain focused on...
FDA Guidance: Many In Vitro DDI Evaluations Should Precede FIH Studies
- Regulatory Guidance
- December 6, 2018
- Dr. Brian Ogilvie, Andrea Wolff
In October 2017, the FDA released its much-anticipated draft guidance documents for drug-drug interaction (DDI) studies, which was finalized in...
Coverage from the October 2018 PBSS DDI Workshop
- Drug Transporters
- November 5, 2018
- Greg Loewen, Michael Millhollen
The Pharmaceutical and BioScience Society (PBSS) hosted the workshop “Drug-Drug Interactions: Update on Risk Assessment, Clinical Evaluation and Regulatory Requirements” on October...
October Presentations on In Vitro Effects of Biologics on CYP Enzymes and Regulatory DDI Guidances
- Regulatory Guidance
- October 26, 2018
- Michael Millhollen
On Oct. 15th at the Peptide ADME Discussion Group Workshop in Gothenburg, Sweden, Dr. Brian Ogilvie presented on In vitro Direct and Cytokine-Mediated...
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
New Recommendation: Evaluate NMEs for OCT1 Transporter-Mediated DDI Potential
- Drug Transporters
- October 1, 2018
- Dr. Brian Ogilvie, Andrea Wolff
Brian Ogilvie, Ph.D., Vice President of Scientific Consulting at XenoTech, presented a case study on the importance of the hepatic OCT1...
Cypex Expands Portfolio of Recombinant Enzymes
- Non-CYP Enzymes
- September 18, 2018
- Scott Hickman
As a distributor for Cypex in the US and Canada, XenoTech is proud to offer this portfolio of products based on patented...
New Rodent, Monkey CryostaX Hepatocytes Featured in Industry News
- Test Systems & Methods
- September 13, 2018
- Michael Millhollen
Following XenoTech’s announcement XenoTech Adds Monkey, Rodent Hepatocytes to Patented CryostaX Product Line, Anticipates the End of Traditionally Cryopreserved Hepatocytes, various...
Challenges & Solutions in Today’s In Vitro Transporter Research
- Drug Transporters
- August 30, 2018
- Michael Millhollen, Dr. Joanna Barbara
Transporters are membrane-bound proteins that govern the passage of drugs into and out of cells. These gatekeeper proteins can be...
XenoTech Named Best Global CRO 2018; Recognised Leaders in Pharmaceutical Safety Testing 2018
- Updated Offerings
- August 24, 2018
- Michael Millhollen
XenoTech has been named the Best Global CRO and was recognized for leadership in Pharmaceutical Safety Testing in the 2018...
Response to FDA Framework for Assessment of Drug-Drug Interactions for Therapeutic Proteins RFI and Comments
- Regulatory Guidance
- August 6, 2018
- Michael Millhollen
In July, Drs. Maciej Czerwinski, Director of Consulting, and Brian Ogilvie, Vice President of Scientific Consulting, submitted XenoTech’s comments in response to the Food...
IQ Induction Working Group Publishes Recommendations for Data Interpretation of In Vitro Induction – Focus on CYP3A4 mRNA
- Enzyme Induction
- July 13, 2018
- Scott Hickman, Dr. Brian Ogilvie
XenoTech is proud to have made contributions to the paper entitled, “Considerations from the IQ Induction Working Group in Response...
New Guidance on Bioanalytical Services
- Regulatory Guidance
- July 6, 2018
- Scott Hickman, Seema Muranjan
XenoTech offers bioanalytical services to support both internal groups and external clients. We have validated LC-MS/MS methods for human CYP markers, UDP...
No-Cost, On-Demand Primary Human Hepatocyte Pooling Using CryostaX Pellets
- Test Systems & Methods
- June 15, 2018
- Michael Millhollen, Dr. Chris Bohl
CryostaX® hepatocytes are created using a patented process that produces unique single-donor cell pellets. This format allows scientists to easily pool primary...
NADPH RapidStart Regeneration System for Extended Metabolism
- Test Systems & Methods
- May 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. NADPH is a critical cofactor...
AAPS-FDA Drug Transporters
- Regulatory Guidance
- May 30, 2018
- Greg Loewen, Michael Millhollen
The American Association of Pharmaceutical Scientists (AAPS) and U.S. Food and Drug Administration (FDA) co-sponsored the workshop “Drug Transporters in ADME:...
In Vitro DDI Regulatory Guidance Reference Poster
- Regulatory Guidance
- May 29, 2018
- Michael Millhollen
At the 2018 Marbach Castle Drug-Drug Interaction Workshop in Germany, Dr. Brian Ogilvie, Vice President of Scientific Consulting, presented a comparison...
Maximize Metabolic Activities by Limiting Hepatocyte Cryoinjury
- Drug Metabolism
- May 1, 2018
- Michael Millhollen, Dr. Chris Bohl
Cryopreservation and thawing of primary hepatocytes causes inherent damage to the cells. Traditional pooling methods require cells to undergo two separate cryopreservation...
Assess Catabolic Stability of Biologics & ADCs with Lysosomes – Characterized Test Systems
- Test Systems & Methods
- April 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. Following up on my series...
No More Water Baths! Simplify Hepatocyte Thaws and Eliminate Contamination
- Test Systems & Methods
- April 3, 2018
- Michael Millhollen, Dr. Chris Bohl
XenoTech has patented technology that eliminates the need for the water baths that are traditionally required to thaw cryopreserved hepatocytes. By removing water...
XenoTech Adds US Support for MATE1 and MATE2-K Drug Transporter Studies
- Drug Transporters
- April 2, 2018
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best In Vitro Drug Metabolism Studies Provider 2018
- Accomplishments
- March 27, 2018
- Michael Millhollen
Following our nomination in late 2017, XenoTech has been named the Best In Vitro Drug Metabolism Studies Provider in the...
Choosing a Relevant Small Animal Model for Pharmacokinetic or Toxicity Studies
- Test Systems & Methods
- March 1, 2018
- Dr. Chris Bohl
Choosing the most suitable small animal model is crucial for pharmacokinetic and/or toxicology studies in order to get usable, relevant data. Due to potential...
Recombinant CYP Bactosomes: Versatile Formats for Many ADME Applications
- Test Systems & Methods
- February 25, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase recombinant CYPs in the EU, please visit tebu-bio’s website. For North American orders, please follow...
Insects and Cattle and Sheep, Oh My!
- Test Systems & Methods
- February 15, 2018
- Michael Millhollen, Aaron Hilgedick
Over the past two decades, we’ve received a lot of interesting requests for custom tissue preparations here at XenoTech. “We’ve...
XenoTech Hiring and Growing to Meet Customer Demand
- Updated Offerings
- January 8, 2018
- Michael Millhollen
As announced earlier this month, XenoTech has hired a record number of new staff over the past year in order to...
What To Do with Microsome Stable Low Turnover Compounds
- Drug Metabolism
- December 7, 2017
- Dr. Chris Bohl
Compounds that exhibit high metabolic stability in hepatocytes and subcellular fractions (S9, microsomes and cytosol) can be a challenge for ADME scientists. These in...
XenoTech Featured in Fall Issue of NewsWave
- Accomplishments
- November 15, 2017
- Michael Millhollen
Leader in pharmaceutical research and scientific collaboration Originally printed in the Fall 2017 Issue of NewsWave. XenoTech, LLC, which is...
Initial Impressions of New Draft FDA DDI Guidance Documents from XenoTech
- Regulatory Guidance
- October 26, 2017
- Michael Millhollen, Dr. Brian Ogilvie
Updated Nov. 6th, 2017 The FDA has released its long-awaited new draft guidance for industry on drug-drug interaction (DDI) studies....
HepatoSure Hepatocytes: The Largest Donor Pool For Your DMPK Applications
- Drug Metabolism
- October 18, 2017
- Isabelle Nobiron
Pooled human hepatocytes can complement a selection of hepatocytes from various species for metabolite profiling, species comparison and evaluation of potential human-specific metabolites. HepatoSure® is the...
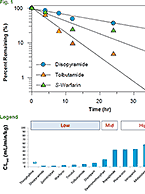
Further Research on the Drug-Drug Interaction Between Gemfibrozil and Repaglinide Presented
- Drug Drug Interactions (DDI)
- September 27, 2017
- Michael Millhollen
The clinically-relevant drug-drug interaction (DDI) between the dyslipidemia drug gemfibrozil and the antidiabetic repaglinide is well-documented throughout the literature. In...
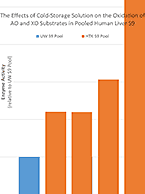
Cold Storage Solution Linked to Lower AO, XO Activity
- Drug Metabolism
- August 22, 2017
- Michael Millhollen
At the time of organ recovery, human livers that have been donated for research are flushed with an ice-cold perfusion...
Considerations In Response to Drug-Drug Interaction Guidances
- Regulatory Guidance
- August 17, 2017
- Michael Millhollen
Check out the recent IQ consortium publication: Considerations from the IQ Induction Working Group in Response to Drug-Drug Interaction Guidances from...
URI Drug Transporters Workshop Presentations
- Drug Transporters
- August 11, 2017
- Michael Millhollen
The University of Rhode Island College of Pharmacy’s 5th Annual Transporters in Drug Discovery and Development: Driving Knowledge from Laboratory...
New Division Directors for Core Services and Logistics
- Updated Offerings
- August 1, 2017
- Michael Millhollen
Following the promotion of Dr. Joanna Barbara, PhD, to Vice President of Scientific Operations, Dr. Etsuko Usuki, PhD, has been promoted...
XenoTech’s Analytical Services Department Adds Instrumentation
- Updated Offerings
- July 17, 2017
- Michael Millhollen
XenoTech’s Analytical Services lab provides researchers with custom method development and method validation, dose solution analysis, in vitro and in vivo metabolite profiling studies, small-molecule non-GLP bioanalysis and...
Considerations When Studying Esterase Activities in the Intestine
- Drug Metabolism
- July 12, 2017
- Dr. Chris Bohl, Michael Millhollen
The gastrointestinal wall is a significant site of first-pass metabolism for oral drugs, which is commonly associated with CYP450 and...
UGT Activities, Concomitant Drugs, and DDI
- Enzyme Induction
- June 20, 2017
- Michael Millhollen
If you have concerns about how your compound may affect UDP-glucuronosyltransferase (UGT) induction and/or inhibition when combined with other therapeutic...
New range of Pig P450s Bactosomes
- Updated Offerings
- June 19, 2017
- Isabelle Nobiron
If you are looking for enzymatic in vitro tools for drug metabolism, reaction phenotyping or metabolite generation using a pig model, you may find these newly...
New Liver Disease Resource and Other Research Biobank News
- Updated Offerings
- May 26, 2017
- Michael Millhollen, Dr. Maciej Czerwinski
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, which affects one out of four people worldwide, such as...
Big Hepatocyte News!
- Test Systems & Methods
- May 10, 2017
- Michael Millhollen
As you may have heard, XenoTech was issued U.S. Patent No. 9,642,355 for the “CRYOPRESERVATION OF CELLS AND SUBCELLULAR FRACTIONS” for its CryostaX® hepatocytes. The...
New VP of Scientific Operations and VP of Scientific Consulting
- Consultancy
- May 4, 2017
- Michael Millhollen
XenoTech has appointed Joanna Barbara, Ph.D., as Vice President of Scientific Operations and Brian Ogilvie, Ph.D., as Vice President of Scientific...
New Lysosome Catabolism Protocol and Tech Tips
- Biologics & Oligonucleotides
- April 17, 2017
- Dr. Chris Bohl
A new guide outlining our IgG Catabolism Protocol as well as Lysosome and Tritosome Technical Tips is now available for those evaluating lysosomal stability...
XenoTech Adds New Drug Transporters to Portfolio
- Drug Transporters
- April 1, 2017
- Michael Millhollen
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of...
XenoTech Named Best for Pharmaceutical Safety Testing 2017
- Accomplishments
- March 2, 2017
- Michael Millhollen
After our nomination in late 2016, XenoTech has been named the Best for Pharmaceutical Safety Testing in the 2017 Biotechnology...
XenoTech Adds New Dermal Subcellular Fraction Test Systems
- Updated Offerings
- February 1, 2017
- Michael Millhollen
XenoTech is adding human and minipig to the company’s list of species with dermal subcellular fractions available as standard test systems for the development...
Pharmaceutical Outsourcing Magazine Interview Nov-Dec 2016
- Updated Offerings
- November 29, 2016
- Michael Millhollen
For their November/December 2016 issue, Pharmaceutical Outsourcing Magazine interviewed Chris Bohl, PhD, Global Technical Support Manager for XenoTech’s Products Division, on the...
XenoTech Standardizes Lysosomal Test Systems for Biopharmaceutical Development
- Test Systems & Methods
- October 5, 2016
- Dr. Chris Bohl
Before releasing these test systems, there was not a high quality, specific, relevant, easy and ready to use, in vitro test system to...
XenoTech Joins Fight Against Liver Disease
- Disease-Specific Resources
- September 16, 2016
- Dr. Maciej Czerwinski, Michael Millhollen
XenoTech is committed to furthering the knowledge surrounding hepatic diseases, such as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis...
XenoTech Featured on Labiotech.EU
- Enzyme Inhibition
- July 5, 2016
- Michael Millhollen
Understand which transporters are involved in a drug’s absorption, distribution & excretion Originally posted on Labiotech.EU How can you build the...
XenoTech Scientists Publish Paper, Present Research Evaluating Ketoconazole and its Alternative Clinical CYP3A4-5 Inhibitors as Inhibitors of Drug Transporters
- Enzyme Inhibition
- April 25, 2016
- Michael Millhollen
XenoTech scientists published a paper in Drug Metabolism and Disposition evaluating Ketoconazole and its alternative clinical CYP3A4-5 inhibitors as inhibitors of drug...
XenoTech Fulfills Largest Order To Date
- Accomplishments
- February 1, 2016
- Michael Millhollen
XenoTech recently fulfilled its largest order of human liver subcellular fractions to date. A large pharmaceutical customer ordered over 30,000...
XenoTech Hosts Bring Your Kids to Work Day
- Our Team
- May 4, 2015
- Matthew Beck, Michael Millhollen
On Thursday, April 23rd, XenoTech participated in “bring your child to work day”. At XenoTech, we are passionate about what...
Navigating the Transporter Wave of 2013
- Regulatory Guidance
- August 28, 2013
- Greg Loewen
The International Transporter Consortium (ITC) issued 7 papers in the July 2013 issue of Clinical Pharmacology & Therapeutics. These 7...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback