

Quality Control is What Makes the Comprehensive Collection of Cell Lines from JCRB Among the Highest Regarded and Most Widely Distributed in the World
- Test Systems & Methods
- April 23, 2019
- Madison Esely-Kohlman, Dr. Arihiro Kohara, Kimiho Yamada
About JCRB cell bank
In addition to focus on preclinical ADME in vitro testing services and complementary products, XenoTech offers to our clients in the academic and industry sectors access to premier cell lines through the Japanese Collection of Research Bioresources (JCRB), a Japanese nonprofit organization founded in 1984. The lines offered span numerous species and can be selected for many different demographic, karyotype, or cell surface markers.
The collection represents over a thousand cell lines with unique qualities including cancer, mutant, genetically-modified, and immortal cells. One featured cell line is HuH7, an immortalized hepatocyte-derived carcinoma cell line widely used in cancer biology studies and developing treatments for Hepatitis C. Because of the high quality and vast selection of the bank, cell lines have been used in cutting-edge research investigating other areas including ovarian cancer and liver cancer. HEC-59, which is a human tumor cell from endometrioid adenocarcinoma G2, has been used by many labs to address oncological research needs not met by other banks.
You can use the Cell Search database on the JCRB website to search for appropriate cell lines by animal, tissue, morphology, cell type, or classification. North American clients can work directly with XenoTech representatives to streamline the order process, making it easy for clients to order their JCRB cell lines without having to worry about shipping and customs.
In addition to variety, quality assurance is a keystone to JCRB, so strict quality control standards for cell lines are maintained in storage and distribution, qualified by testing for microbial, virus, or cross-cultural contamination.

Cell bank features
- Over 1,600 human and animal cell lines
- Human culture cells derived from various disease patients
- Immortalized human mesenchymal stem cell lines
- More than 4,600 JCRB cell lines distributed all over the world per year
Quality control
Quality control is paramount in the storage and distribution of cell lines, for both reproducibility of results and safety assurance. All cell lines distributed from the JCRB Cell Bank have been confirmed as contaminant-free and authenticated through the quality control measures listed below. The data obtained from the quality control testing for each cell line is used to continually update the JCRB website.
- Bacteria and fungi testing
- Mycoplasma screening
- Cell line authentication
- Viral examination
- Species identification
- Chromosome analysis
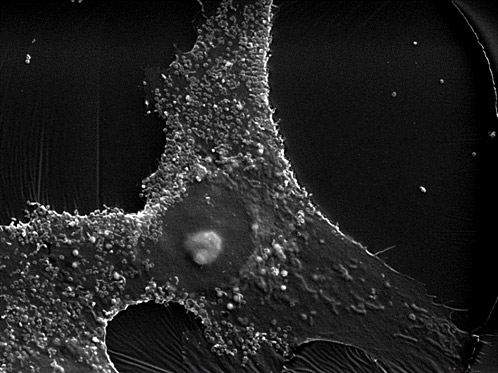
1. Bacteria and fungi testing
To assess contamination of bacteria and fungi in cell lines, cells are disseminated to three kinds of media optimized for bacteria and fungi.

1 day after finger touch
3 days after sneeze
Media : Blood agar with 5% rabbit blood
Nutrient broth with 2% yeast extract (NB medium) Thioglycolate broth (TGC medium)
Cultivation temperature: 35°C
Cultivation period: 1 week – 2 months (depending on medium)
2. Mycoplasma screening
Mycoplasma contamination is a serious problem that can lead to erroneous data and cause unnecessary repetition of experiments. Our routine procedure to assess mycoplasma contamination consists of the following three methods:
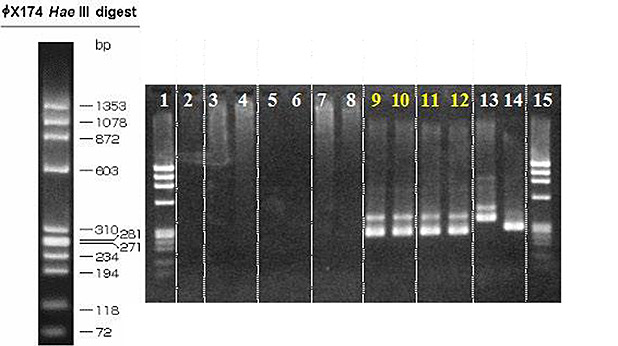
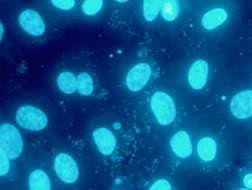

Exploits the activity of mycoplasmal enzymes
3. Cell line authentication
STR (short tandem repeat) analysis is a common method for authentication of human cell lines. STRs are repetitive sequence elements 2–7 base pairs in length, widespread throughout the human genome. The polymorphisms in STRs are due to the different number of copies of the repeat element that can occur in a population. JCRB scientists analyzed 9 or 16 STR loci with our human cell lines and have developed a database of STRs.
Step 1 DNA extraction from cell lines by using FTA Eluate
Step 2 STR-PCR analysis by using PowerPlex® 16 HS system
| Fluorescent Dye | Loci |
| fluorescein | D3S1358, TH01, D21S11, D18S51, Penta E |
| Rhodamine | Amelogenin, vWA, D8S1179, TPOX, FGA |
| JPE | D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta D |
Step 3 Analysis of the STR profiles
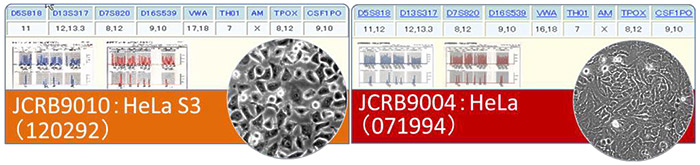
The search will pick up one STR profile datum of the particular cell from the JCRB profile database. The system will then calculate an evaluation value (EV) against all other human cells in the JCRB profile database.
| EV= | (Number of coincidental peaks) x 2total numbers of peaks in a cell A + total numbers of peaks in a cell B |
Comparison with the data of 11,480 cell lines and Hela cells

When arbitrary two cells are compared and the EV is 0.85-1.00, it is determined that these two cell lines are derived from the same origin.
The reason why EV≠1.0
- Genetic instability of cancer cells
- Cultured cells are heterogeneous
- Repeated DNA sequences by the lack of repair enzymes
4. Viral examination
Human cell lines are screened for 20 pathogenic viruses by targeting viral DNA and/or RNA fragments. JCRB established virus examination system for the first time in cell banks.
DNA viruses
CMV, EBV, HHV-6, HHV-7, BKV, JCV, ADV, HBV, ParvoB19, PV18
RNA viruses
HAV, HCV, HTLV-1, HTLV-2, HIV-1, HIV-2

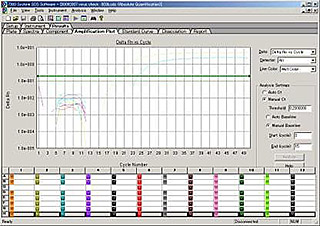
5. Species identification
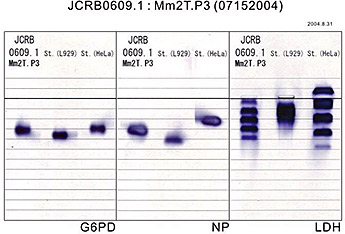
Animal cell lines can be identified for their origin of species by isozyme and PCR analysis.

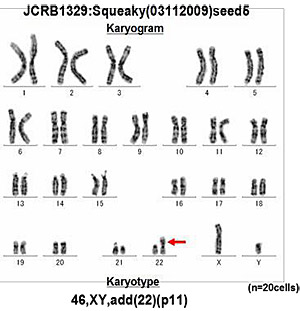
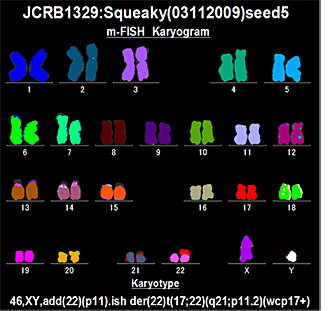
6. Chromosome analysis
The number of chromosomes is counted by Giemsa-stained metaphases, which reveals differences between cells in the heterogeneous population. Karyotyping is performed by G- banding, which identifies chromosome rearrangements characterizing each cell line (these data are limited to certain cell lines).
Learn more about:
About the Authors
Related Posts
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
