

What To Do with Microsome Stable Low Turnover Compounds
- Drug Metabolism
- December 7, 2017
- Dr. Chris Bohl
Compounds that exhibit high metabolic stability in hepatocytes and subcellular fractions (S9, microsomes and cytosol) can be a challenge for ADME scientists. These in vitro drug metabolism test systems are easy to source and use at the bench, and the methods for xenobiotic metabolism in these matrices are largely standardized and accepted by the scientific community. However, these systems are also limited to a 4-6 hour window of time when they are metabolically active and can generate quality data.
There have been many interesting advances in the field to address this constraint, however the complexity, amount of labor and cost involved can make these procedures difficult to establish in your lab. Scientists at XenoTech have developed and patented CryostaX® technology, which combines ease of use and cost effectiveness for examining low-turnover compounds in house. Supported by XenoTech’s optimized medium, pooled, plateable human hepatocytes can be cultured for up to 48 hours without a medium change; giving a significantly extended incubation window in which to collect metabolism data (Fig. 1). When compared to alternative methodologies used to assess low-turnover compound metabolism, CryostaX® generates comparable metabolism data in a format that is easier to use and more cost effective. (Fig. 2).
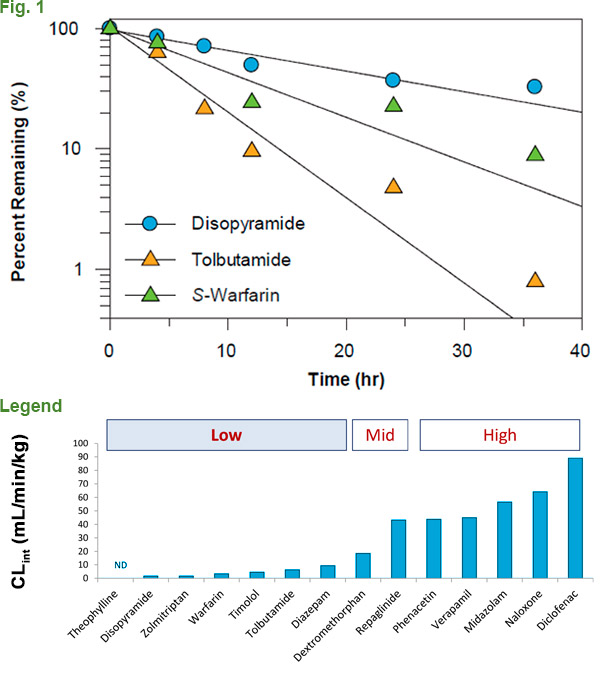
Legend. Clint values for 14 compounds with CryostaX®
pooled plateable hepatocytes.
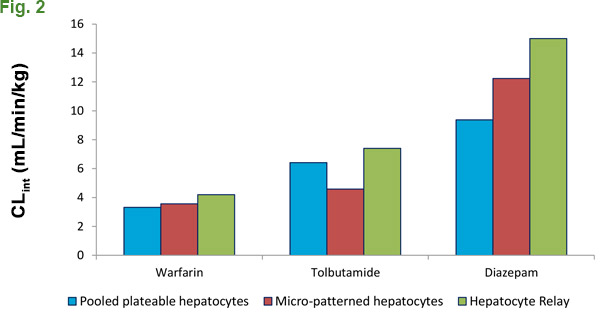
We successfully used plated hepatocytes to measure substrate loss and calculate intrinsic clearance, even for notoriously low-turnover small molecule drugs. Some groups have not been able generate significant data using other protocols and methods,1 but we’ve created a custom pool to recapitulate our results.
CryostaX® technology enables XenoTech’s scientists to create attaching, customizable pools of hepatocytes that have only undergone a single cryopreservation cycle, versus the two cycles that are required for traditional pooling methods, thereby protecting the cells’ drug metabolizing activities by minimizing the cryoinjury brought on by each round of cryopreservation. Talk to a representative of our Products Team to find out more.
Learn more about:
1Hutzler, J. M., et al. “Low-Turnover Drug Molecules: A Current Challenge for Drug Metabolism Scientists.” Drug Metabolism and Disposition, vol. 43, no. 12, 2015, pp. 1917–1928., doi:10.1124/dmd.115.066431.
About the Authors
Related Posts
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
