Upcoming & Archived Events
RI-ADE ADME 101: Non-clinical pharmacokinetics studies using radio-labeled compounds
Presenter: Satoshi Ito, Drug Development Solutions Center ADME Group Manager, Sekisui Medical Radio-labeled compounds can be used to provide important information...
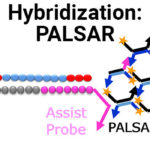
Dual-Hybridization PALSAR (Probe Alternation Link Self-Assembly Reaction) Technology Introduction
Presenter: Yuya Ono, MSc, MBA. Sekisui Medical, Co. Ltd. The quantitative bioanalysis of oligonucleotide therapeutics is mainly conducted with LC-MS/MS and...
Webinar: Design Intricacies and Decision-Making Strategy for DDI Studies
Hear drug-drug interaction (DDI) expert, Dr. Andrew G. Taylor, discuss how to avoid common pitfalls by tweaking ADME/DDI strategies to...
XenoTalks™ Seminar Series: The Importance of ADME and DDI in Drug Development
Event Details: These fully interactive events provide in-depth presentations from industry experts along with free food and drinks and good...
Webinar: New 2022 Draft ICH Drug Interaction Studies Global Guideline
Presenter: Brian Ogilvie, Ph.D, Vice President of Scientific Consulting at XenoTech Drug-drug interactions (DDI) may occur when taking two (or...
2022 ISSX/MDO Meeting
We’re looking forward to the ISSX/MDO Meeting in Seattle on Sept. 11-14, including the corresponding Hepatocyte Research Association Meeting at...
Webinar: Role of Sulfotransferases in Drug Metabolism and Potential for Drug-Drug Interactions
Register to hear expert insights on the importance of SULTs in drug metabolism and DDI, tissue distribution and gene regulation...
2022 SOT Annual Meeting and ToxExpo
This year’s meeting will take place in-person in San Diego, CA. You can view more details at the event website....
Webinar: Bridging In Vitro Data to Clinical Outcomes with MetID
Presenters: Andrew G. Taylor, Ph.D., Manager of Technical Support for Services at SEKISUI XenoTech and Heather Heading, Director of LCMS at Agilex...
2021 Holiday Wishes
From Our “Culture” to Yours! 😉 Best Wishes and Season’s Greetings from Your Team at XenoTech! Listen to the 2020...