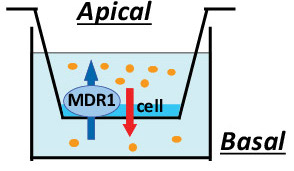
Transwell Drug Transport Assay Design
Our transwell assay allows the evaluation of drug transport across efflux transporter-expressing cells, using MDCKII cells cultured on a transwell plate.
Transcellular (Bi-Directional) Drug Transport
MDR1 (P-gp) and BCRP are important efflux transporters involved in limiting the oral bioavailability of compounds, limiting the penetration of compounds through the Blood-Brain Barrier (BBB), and in the excretion of compounds in the urine and bile. MDR1, and to a lesser extent BCRP, has been implicated in numerous drug-drug interactions. Interactions of compounds with MDR1 and BCRP are evaluated by measuring transcellular (bi-directional) transport of the compound or a probe substrate across MDR1 or BCRP transfected MDCKII and control cells. Transfected and control cells are cultured on a transwell plate. Experiments can also be performed in Caco-2 cells.
Efflux Transporter Substrate Potential
The test compound is added to either the apical or basal side of the monolayer and the amount of compound permeating through the cell monolayers is measured by LC-MS / MS or liquid scintillation counting. The apparent permeability (Papp) of the compound is calculated in both the apical to basal and basolateral to apical directions (hence the term bidirectional permeability). The efflux ratio of permeability (B to A/A to B) is calculated. MDR1 and BCRP pump in the B to A direction; therefore, substrates for these transporters exhibit a greater permeability in the B to A direction than the A to B direction. This results in an efflux ratio greater than 2.

Efflux Transporter Inhibition Potential
The ability of the test compound to inhibit the bi-directional drug transport of a probe substrate (e.g. digoxin for P-gp or prazosin for BCRP) is measured. The probe substrate is added to either the apical or basolateral side of the cell monolayer and test compound is added to both sides. The inhibitory effect of the drug candidate is evaluated by measuring the efflux of the probe substrate in the presence of multiple test compound concentrations in both directions. These data can then be used to calculate an IC50 value (concentration causing 50% inhibition of transporter-mediated transport).
Related Resources
For more information to guide your decision making on timing and individual transporters to assess, access our Drug Transporters Decision Tree, a poster comparing of regulatory expectations between FDA, EMA, and PMDA or our collection of transporter-related scientific content. Additional features of our drug transport study design and a chart of known clinically relevant transporter DDIs can be found on our Drug Transport Services page.