Our partners at the Drug Development Solutions Center offer a receiver operating characteristics (ROC) analysis as a screening service to predict a drug candidate’s likelihood to cause cholestasis or other drug induced liver injury-related endpoints.

Receiver Operating Characteristic (ROC) Analysis to Estimate Risk of Clinical DILI
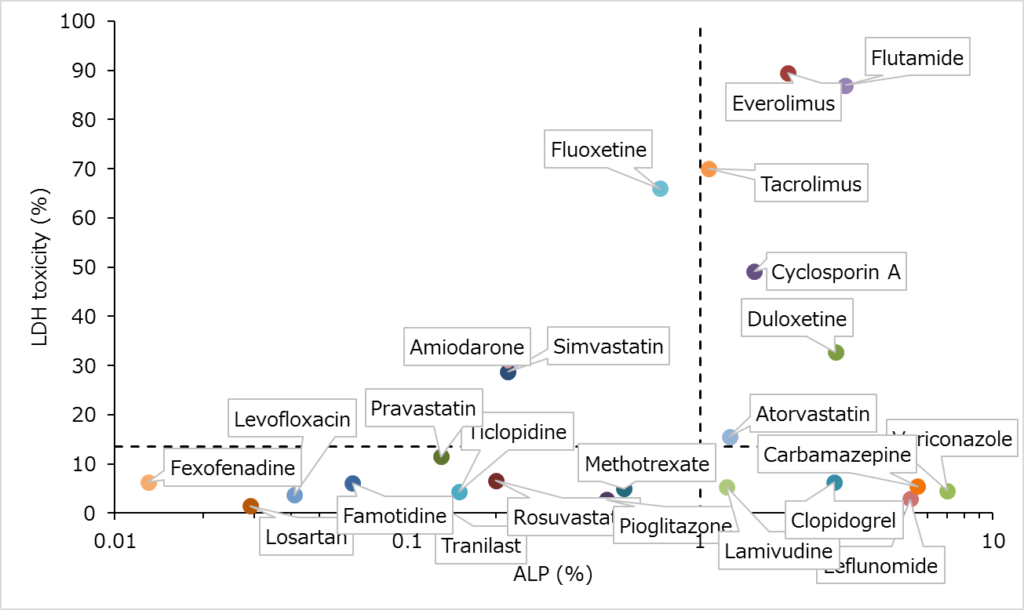
ROC Analysis creates a plot comparing true positive (sensitivity) rate to false positive (specificity) rate when the discrimination threshold is varied.
Our ROC service will incorporate values from multiple compounds comparing lactate dehydrogenase (LDH) release to alkaline phosphatase (ALP), a known clinical hepatotoxicity biomarker. The cut-off value can be calculated from clinical data (increasing rate of ALP) of various compounds and the LDH release rate obtained in in vitro cytotoxicity experiments to predict clinical DILI risk associated with the test article.
The in vitro data comes from an abbreviated version of our full Bile Acid-Induced Hepatotoxicity functional assay. Cultures of single-donor primary human hepatocytes are treated with test article +/- bile acid mixture and LDH release is measured. Experiments are performed in triplicate, for one concentration each of the drug candidate and 24 compounds with variable levels of known clinical degrees of DILI incidents (indicated by ALP, a common clinical biomarker of hepatotoxicity). For more information on the study design of the functional assay alternative to this screening service that gives more insight to concentration-dependence, see our page on Bile Acid-Induced Hepatotoxicity.
This analysis will illuminate effects of all types of hepatotoxicity caused by the drug candidate. Some hepatotoxic drugs show toxicity in the cultures without the bile acid mixture, others only induce hepatotoxicity in the presence of the bile acid mixture. These can be important distinctions to a drug developer in understanding the mechanisms of toxicity of their drug candidate.

