
Placental Transfer Studies to Support In Vivo Distribution Data
Feto-placental transfer and lacteal transfer studies are typically performed together with toxicity studies as a part of the development of drugs. For women of child-bearing potential these studies may be conducted to determine the potential exposure and risk to the fetus and breastfeeding infant.
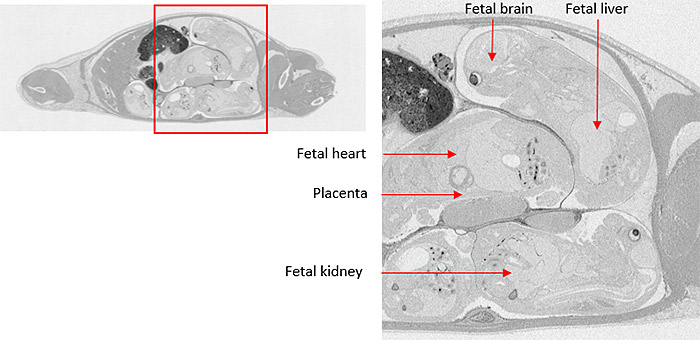
A tissue distribution study (tissue dissection and/or whole body autoradiography) using pregnant rats at the organogenesis period and/or perinatal period, is conducted to elucidate maternofetal transfer of a radiolabeled drug compound in various dosing routes (see table below). A lacteal (breastmilk) transfer study involves treatment of lactating rats with test article and measurement of drug-related material with Liquid Scintillation Counter (LSC) analysis.
Our Approach to Placental Transfer Studies for In Vivo ADME
Placental transfer and lacteal transfer studies are included in our specialized in vivo ADME offerings performed by our partners. Our partner in Japan, the Drug Development Solutions Center, has accumulated over 50 years’ experience performing radiolabeled (RI) experimentation, synthesis, and purity checks for pharmaceutical companies as the leading in vivo ADME CRO in Japan. The facilities are AAALAC-accredited to meet compliance with ethical requirements upheld by FDA, EMA, and PMDA for all animal studies, radiolabeled compound synthesis and related in vivo ADME capabilities.
A feto-placental transfer study is similar to a QWBA or tissue dissection study; animals are administered radiolabeled test article during the organogenesis period and/or perinatal period, and distribution data are obtained using the QWBA method or LSC. A lacteal transfer study is more comparable to an in vivo pharmacokinetic (PK) study; after dosing lactating animals with radiolabeled test article, milk is collected at serial time points and radioactivity concentration is quantified using LSC analysis.
In Vivo ADME Study Capabilities
| HUMAN CYP MARKERS | UGT, MAO AND OTHERS | ANIMAL P450S | DRUG TRANSPORT |
|---|---|---|---|
| 1′ -Hydroxy midazolam (3A4) Oxidized nifedipine (3A4) | Estradiol-3-β-D- glucuronide (UGT1A1) Trifluoperazine glucuronide (UGT1A4) | Rat Hydroxy- testosterone (2α, 6β, 7α, 16β) Mouse Hydroxy- testosterone (6β, 15α, 16β) | Digoxin (MDR1) |

