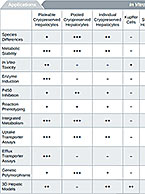
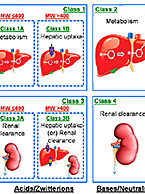
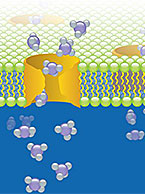
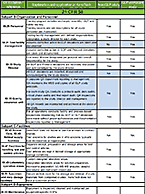
Drug-drug interactions (DDI) can be fatal or harmful to a patient taking multiple medications by causing up-regulation or inhibition of drug-metabolizing enzymes or altering activity of drug transporters. Understanding DDI potential of an investigational new drug before clinical trials can help bolster safety data and evaluate risk. To learn more about why DDI potential of a compound is important or to contract relevant studies, visit our Drug-Drug Interactions (DDI) informational page.