News
May 1st Transition of XenoTech.com Products and Services to BioIVT.com
Since XenoTech joined BioIVT in Sept. 2022, we have been working to migrate all of the products and services to...
XenoTech Joins BioIVT: Expands Biospecimens Portfolio and Research Services
BioIVT, a leading provider of research models, biospecimens and research services for drug discovery and development has acquired XenoTech Along...
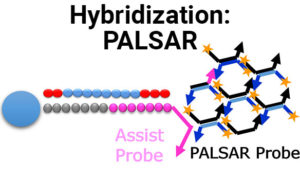
New PALSAR Method Now Available for Bioanalysis of Oligonucleotides and Other Intermediate Molecules
We are proud to announce our newest bioanalytical method, PALSAR, which is a proprietary hybridization method for the measurement of oligonucleotides, ADCs and other intermediate…
New Efficiencies Lower Study Timelines While Maintaining Quality Standards
Following improvements to our Electronic Lab Notebook (ELN) system, XenoTech is experiencing increased efficiency across our portfolio of ADME/DMPK and...
Increased Access to OATP2B1 Drug Transporter Studies
Evaluating drug transporters during the drug development process is of critical importance because they assist in the movement of drugs...
Reduction of Energy a Priority at XenoTech
As part of our ongoing Sustainable Development Goals (SDGs), we are always looking for opportunities to improve. From considering larger...
New Cysteine Trapping Study Now Available for More Efficient & Effective Early Phase Drug-Induced Liver Injury (DILI) Evaluation
DILI is a leading cause of adverse events in clinical trials, so our partner in Japan, SEKISUI, offers a variety...
XenoTech expands access to Microsomal Protein Binding service for DMPK profile development and DDI assessment of new drugs
To help customers more easily investigate how much of their drug will be available to bestow its intended therapeutic effect, XenoTech is now offering Microsomal…
XenoTech expands access to Red Blood Cell Partitioning service for improved pharmacokinetics assessments
To help customers better investigate their compounds’ clearance in preclinical drug development, XenoTech has expanded in vitro service capabilities of...
Introducing New CryostaX Individual Donor Human Hepatocytes
Using single donor hepatocytes just got a whole lot easier. We’ve just released new Single Donor CryostaX®. If you’ve used...