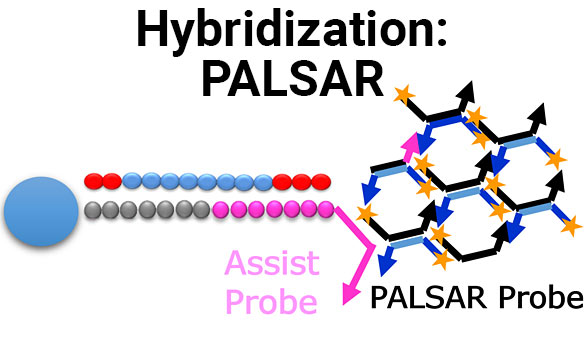
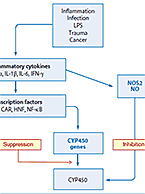
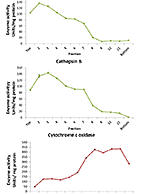
Biologic drugs include monoclonal antibodies, hormones, growth factors, enzymes, vaccines, oligonucleotides and other large molecule therapeutics. While the evaluation of drug-drug interactions (DDIs) with small molecule drugs is well established, it is an area of growing concern and investigation for biologic drugs. Our in-house research and development teams have presented research and developed assays to explore specific intersections of biologics and small molecules in the context of DDI. For more information about these services, visit our Biologic-Small Molecule DDI page.