
In Vivo Tissue Distribution Studies for ADME in Drug Development
You can now request quotes for our research services on BioIVT.com!
Whether you need a single assay or a complete ADME program, BioIVT’s experts will help design and implement the appropriate studies for your drug and research objectives. View BioIVT’s comprehensive portfolio of ADME research services.
Distribution is a key component of uncovering absorption, distribution, metabolism, and excretion (ADME) and pharmacokinetic properties of xenobiotics, investigating the arrival of a drug to its target location and accumulation in various tissues and organs following administration. A drug compound’s distribution can be affected by multiple factors, including plasma protein binding or lysosomal trapping, and needs to be measured accurately and appropriately in accordance with regulatory requirements. Whole-body distribution studies also yield key data for planning first in-human (FIH) mass balance studies in the clinic.
Tissue Distribution Services to Develop Your Drug’s Pharmacokinetic Profile
Quantitative Whole-Body Autoradiography (QWBA)
Quantitative whole-body autoradiography is an in vivo study method in which a rat (or in some cases mouse) is dosed with radiolabeled test article and at successive time points radioactivity is measured in cross-sectioned slides of the whole animal to show distribution over time.
Tissue Dissection
Tissue dissection can supplement QWBA data by quantifying a radiolabeled compound’s distribution in a particular organ at successive time points, allowing further investigation of accumulation in substructures or highly-localized tissue types.
Micro-Autoradiography (mARG)
Micro-Autoradiography is a technique which allows an even finer scale of distribution analysis, qualitatively measuring localization of radiolabeled parent drug or its metabolites at a cellular level.
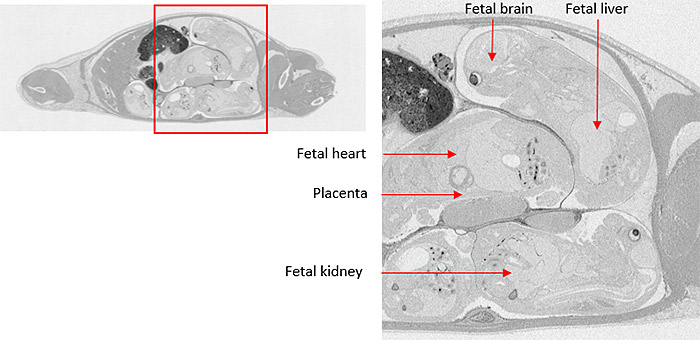
Placental Transfer
Single-dose in vivo placental transfer studies administer radiolabeled compound to the mother, followed by collection of biological matrices (i.e. maternal blood, amniotic fluid, placentas, and/or fetuses) at multiple time points post-dose.
Our Approach to In Vivo ADME
The in vivo distribution services available through our partners use radiolabeled compounds to give whole-body or organ-specific distribution data because radioactivity is a highly sensitive way to quantitatively assess the localization of a parent molecule or its metabolites and required by regulatory agencies prior to clinical trials. With more than 50 years of experience, our partners have unparalleled expertise in radioisotope (RI) synthesis and radiolabeled in vivo ADME/PK services to provide drug developers with an experienced team to get the most accurate data with fast turnaround times and carefully planned and executed nonclinical studies.
Regulatory Compliance
Studies conducted with our partners in Japan also meet or exceed standards required by regulatory authorities, including FDA and EMA, just as with our Kansas City campus. While in the United States, there is no method by which an institution can claim certified compliance with Good Laboratory Practice (GLP) standards, the PMDA can grant certification following an inspection that approves a Japanese facility as GLP-compliant in specific practices. Their Drug Development Solutions Center has this certification for all bioanalytical services, a critical component of many animal studies. The Center is also certified by AAALAC International for ethical use of animals in research so you can be sure services exceed quality standards required by the FDA upon IND or NDA submission.
By contracting a Drug Development Solutions Center study through XenoTech, we make it easy for North American and European clients to benefit from the years of experience our Tokai team has built. Read more in our blog about how we make it easy to work with the Drug Development Solutions Center for your in vivo nonclinical services.
