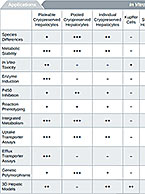
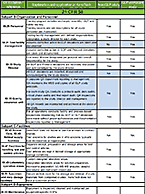
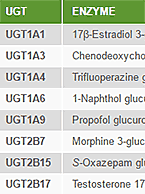
Enzyme inhibition studies evaluate a compound’s inhibitory effect on drug-metabolizing enzymes to predict the potential for drug-drug interactions that may increase toxicity or reduce therapeutic effect of concomitant medications. To learn more about this service, visit our Enzyme Inhibition contract service page.