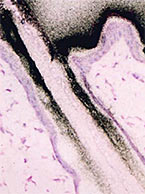
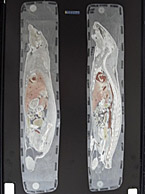
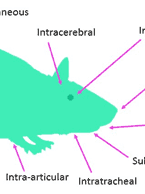
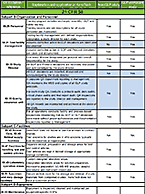
In vivo and radiolabeled compound studies are necessary for distribution data, dosing calculations for first in-human studies, bridging in vitro PK and toxicology data, and much more. Find resources here relating to our in vivo and radiolabeling services offered byt the Drug Development Soluctions Center. The Center offers GLP and non-GLP animal studies to investigate your drug’s pharmacokinetics as well as top-tier radioisotope (RI) compound labeling services for pharmaceuticals. For more information about these services, visit our In Vivo ADME/PK page.