Biologics & Oligonucleotides
Can Interactions Between Therapeutic Proteins and Small Molecule Drugs Be Evaluated In Vitro?
- Biologics & Oligonucleotides
- April 4, 2019
- Greg Loewen, Dr. Maciej Czerwinski
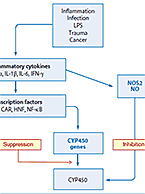
The mechanisms of clearance for small molecule drugs and therapeutic proteins are fundamentally distinct and therefore therapeutic proteins are generally not...
October Presentations on In Vitro Effects of Biologics on CYP Enzymes and Regulatory DDI Guidances
- Regulatory Guidance
- October 26, 2018
- Michael Millhollen
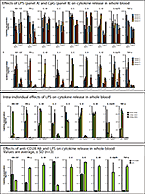
On Oct. 15th at the Peptide ADME Discussion Group Workshop in Gothenburg, Sweden, Dr. Brian Ogilvie presented on In vitro Direct and Cytokine-Mediated...
Response to FDA Framework for Assessment of Drug-Drug Interactions for Therapeutic Proteins RFI and Comments
- Regulatory Guidance
- August 6, 2018
- Michael Millhollen
In July, Drs. Maciej Czerwinski, Director of Consulting, and Brian Ogilvie, Vice President of Scientific Consulting, submitted XenoTech’s comments in response to the Food...
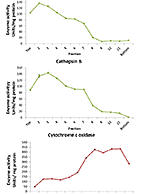
Assess Catabolic Stability of Biologics & ADCs with Lysosomes – Characterized Test Systems
- Test Systems & Methods
- April 30, 2018
- Isabelle Nobiron
Originally published in tebu-bio’s Being Bioreactive. To purchase XenoTech’s products in the EU, please visit tebu-bio’s website. Following up on my series...
New Lysosome Catabolism Protocol and Tech Tips
- Biologics & Oligonucleotides
- April 17, 2017
- Dr. Chris Bohl
A new guide outlining our IgG Catabolism Protocol as well as Lysosome and Tritosome Technical Tips is now available for those evaluating lysosomal stability...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback