Author: Madison Esely-Kohlman
DDI & Drug Repurposing Article featured in Drug Discovery World (DDW) Spring Edition 2021
- Drug Drug Interactions (DDI)
- April 29, 2021
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Repurposing (repositioning, re-profiling, or re-tasking) a drug potentially saves years of costly testing from going to waste and potentially providing a higher chance of success.…
Meet the Scientist: Stephanie Helmstetter
- Meet the Scientist
- February 20, 2021
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable product experts, Stephanie Helmstetter.
Interview with Outsourcing Pharma: Repurposing existing drugs accelerates discovery
- Consultancy
- January 18, 2021
- Madison Esely-Kohlman
“Exploring alternative uses for drugs tapped for other indications, can save considerable time and money in discovery, according to an...
Meet the Scientist: Lois Haupt
- Meet the Scientist
- November 6, 2020
- Madison Esely-Kohlman
As part of our Meet the Scientist series, we introduce you to one of our most notable Enzyme Inhibition experts, Lois Haupt.
Updated Look & Fresh Features: Welcome to Our Brand New Website!
- Updated Offerings
- September 18, 2020
- Madison Esely-Kohlman
We are thrilled to welcome you to the brand new XenoTech website! The reconstruction project is a massive overhaul of over 1,000 pages carefully recrafted…
Drug-Drug Interaction (DDI) Prediction Models Following In Vitro Studies in Preclinical Development
- Drug Drug Interactions (DDI)
- August 12, 2020
- Madison Esely-Kohlman, Dr. Pallavi Limaye, Andrea Wolff, Dr. Maciej Czerwinski
In preclinical development, a drug will be evaluated for potential to cause a drug-drug interaction (DDI) using in vitro experiments and then calculations that...
Toxicokinetic (TK) Analysis for Preclinical Drug Development
- In Vivo & Radiolabeling
- August 6, 2020
- Madison Esely-Kohlman, Jolanta Golec, Dr. Pallavi Limaye
The main goal of preclinical toxicokinetic (TK) studies is to establish a correlation between a candidate compound’s concentration or dose...
Important DDI Considerations for Repurposing Drugs to Treat COVID-19
- Drug Drug Interactions (DDI)
- June 8, 2020
- Madison Esely-Kohlman
“Given the rapid spread of COVID-19 and its relatively high mortality, filling the gap for coronavirus-specific drugs is urgent. […]...
What is DMPK and how does it fit into drug development?
- Drug Metabolism
- May 11, 2020
- Madison Esely-Kohlman
Drug metabolism and pharmacokinetics (DMPK) is a core discipline in drug development that considers the biotransformation of a drug compound...
Meet the Scientist: Pallavi Limaye
- Meet the Scientist
- April 28, 2020
- Madison Esely-Kohlman
We have welcomed a new consultant to the team! Dr. Pallavi Limaye now serves as a Director in the Scientific...
ADME and Drug-Drug Interactions for the Toxicologist
- Drug Drug Interactions (DDI)
- April 28, 2020
- Madison Esely-Kohlman
Highlights from the recent webinar presented by our newest expert consultant, Dr. Pallavi Limaye In her recent webinar (now available for...
New TMPRSS2-Expressing Cell Line for COVID-19 Research
- Updated Offerings
- April 23, 2020
- Madison Esely-Kohlman
We are offering a brand new cell line to assist researchers in the fight against COVID-19.The cell line (JCRB1819), developed...
What is ADME and how does it fit into drug development?
- Drug Metabolism
- April 10, 2020
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The main aim of drug development is to get a compound that has a therapeutic effect into the form of...
In Vitro Evaluation of Drug-Drug Interaction (DDI) Potential
- Enzyme Inhibition
- March 24, 2020
- Madison Esely-Kohlman, Zachary Mitts, Dr. Joanna Barbara
In its most recent in vitro drug interaction guidance update, the US Food and Drug Administration (FDA) emphasized harmony with the...
Four ways to optimize preclinical in vitro data to mitigate risk of late-stage clinical failure
- Consultancy
- March 9, 2020
- Madison Esely-Kohlman
1. Collect high-quality data to make informed, confident go/no go decisions for moving your drug candidate forward If you need...
How can in vitro and in vivo studies help me understand my drug’s clearance?
- Regulatory Guidance
- March 5, 2020
- Madison Esely-Kohlman, Dr. Joanna Barbara
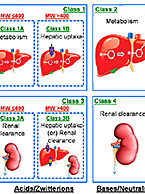
Systemic clearance, denoting how much drug is cleared from a volume of blood per unit of time, is of critical...
Timing of In Vitro Studies: Early, Thorough ADME for Your Compound’s Success
- Regulatory Guidance
- February 13, 2020
- Madison Esely-Kohlman, Dr. Chris Bohl, Greg Loewen
Beyond the need for evidence that a drug works, Food and Drug Administration (FDA) and other regulatory bodies around the...
How Can I Make Sure My Data Meets Regulatory Expectations?
- Regulatory Guidance
- January 10, 2020
- Madison Esely-Kohlman, Greg Loewen
Regulatory authorities publish updated guidance documents that share their expectations for endpoints and test systems with drug developers, but sometimes it is difficult to...
What In Vitro Metabolism and DDI Studies Do I Actually Need?
- Regulatory Guidance
- November 25, 2019
- Madison Esely-Kohlman, Greg Loewen
Though there is no ‘roadmap’ spelling out required studies to achieve regulatory approval for clinical entry, a drug candidate’s metabolism...
I Have My Data… Now What?
- Consultancy
- October 16, 2019
- Madison Esely-Kohlman
As a drug moves through the development pipeline, it undergoes a rigorous battery of safety assessments to prove it will...
The FDA has requested follow-up data… how do I fill in the gaps?
- Regulatory Guidance
- October 9, 2019
- Madison Esely-Kohlman
Because each new drug is unique in characteristics, such as chemical structure, mechanism of action, and physicochemical properties, there can...
Cypex Adds Recombinant UGTs
- Updated Offerings
- September 3, 2019
- Madison Esely-Kohlman
Recombinant enzymes represent a useful test system for studies requiring extra high levels of catalytic activity. Due to high customer demand, Cypex has...
Consultancy Expansion
- Consultancy
- August 20, 2019
- Madison Esely-Kohlman
We are proud to house some of the brightest minds in this field who have seen “the weird stuff” when...
Reduced Pricing for In Vitro Transporter Studies
- Updated Offerings
- August 20, 2019
- Madison Esely-Kohlman
We’re pleased to announce that our scientists’ ongoing dedication to enhancing our processes and discovering new efficiencies without sacrificing quality...
In Vivo ADME: What You Need and Why You Need It
- In Vivo & Radiolabeling
- July 22, 2019
- Madison Esely-Kohlman, Satoshi Ito
When putting together a data package for regulatory approval by the FDA, EMA, or PMDA, there is a lot to...
The Story of Us
- Updated Offerings
- July 1, 2019
- Madison Esely-Kohlman, Satoshi Ito
This year at XenoTech we celebrate our 25th birthday, and our partner in Japan turns 64! Looking back over our history,...
Transporters of Emerging Importance in Drug Development: Beyond the Guidance Documents
- Regulatory Guidance
- June 25, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Dr. Ogilvie’s presentation discusses critical literature and case studies which have been published following the FDA’s 2017 guidance revision, and covered...
In Vitro Induction Studies: Elements of Design and Important Considerations in Data Analysis
- Enzyme Induction
- May 13, 2019
- Madison Esely-Kohlman, Dr. Joanna Barbara, Rebecca Campbell
Why do induction studies? Induction potential is an important piece of the drug-drug interaction (DDI) component of an IND submission. Simply put, we...
Official PMDA English Translation
- Regulatory Guidance
- May 7, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The Japanese regulatory agent PMDA (Pharmaceuticals and Medical Devices Agency) recently published an official English translation of their final Drug Interaction Guideline...
Quality Control is What Makes the Comprehensive Collection of Cell Lines from JCRB Among the Highest Regarded and Most Widely Distributed in the World
- Test Systems & Methods
- April 23, 2019
- Madison Esely-Kohlman, Dr. Arihiro Kohara, Kimiho Yamada
About JCRB cell bank In addition to focus on preclinical ADME in vitro testing services and complementary products, XenoTech offers to our clients...
Minding Your Binding: Plasma Protein Binding Potential Study Now Available at Our US Labs
- Test Systems & Methods
- April 15, 2019
- Madison Esely-Kohlman, Andrea Wolff, Dr. Joanna Barbara
While liver and intestine are important in ADME, behavior in blood is also crucial. When an orally administered drug or...
Studies in Japan: Easier & More Beneficial than You Might Think
- In Vivo & Radiolabeling
- April 8, 2019
- Madison Esely-Kohlman
XenoTech is well-known to pharmaceutical companies and toxicology academics alike for unparalleled experience in quality in vitro ADME/DMPK/DDI studies and complementary products,...
New Liver Tissue Microarrays Available – What Would Benefit Your Research?
- Disease-Specific Resources
- April 2, 2019
- Madison Esely-Kohlman, Dr. Maciej Czerwinski
As technology evolves to better meet needs in exploration of drug metabolism pathways and disease mechanisms, familiarization with appropriate and...
Four Ways CROs Drive Innovation for Improved In Vitro Drug Metabolism and Pharmacokinetics (DMPK) Studies
- Drug Metabolism
- May 17, 2021
- Darina Hynes, Madison Esely-Kohlman
Agility and focused growth allow drug developers to outsource expertise and benefit the industry at-large by fostering innovation. Panelists at the 2021 Annual DMDG Meeting…
DDI & Drug Repurposing Article featured in Drug Discovery World (DDW) Spring Edition 2021
- Drug Drug Interactions (DDI)
- April 29, 2021
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Repurposing (repositioning, re-profiling, or re-tasking) a drug potentially saves years of costly testing from going to waste and potentially providing a higher chance of success.…
Important DDI Considerations for Repurposing Drugs to Treat COVID-19
- Drug Drug Interactions (DDI)
- June 8, 2020
- Madison Esely-Kohlman
“Given the rapid spread of COVID-19 and its relatively high mortality, filling the gap for coronavirus-specific drugs is urgent. […]...
Missing Out on Annual ‘Comforting of the Soul’
- Our Team
- May 29, 2020
- Deja Coffin, Madison Esely-Kohlman, Jolanta Golec
As we begin this summer, many of us are grieving opportunities lost for parties and pool days with friends and...
What is ADME and how does it fit into drug development?
- Drug Metabolism
- April 10, 2020
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The main aim of drug development is to get a compound that has a therapeutic effect into the form of...
What You Need to Know About Micro-Autoradiography (mARG) Distribution Studies
- In Vivo & Radiolabeling
- December 19, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo determination of drug localization in tissue can be uniquely informative to drug developers investigating distribution within the context of...
Mass Balance Studies: What You Need and Why You Need It
- In Vivo & Radiolabeling
- November 15, 2019
- Jolanta Golec, Madison Esely-Kohlman
In vivo mass balance studies are an important element of nonclinical drug development, to inform first in-human (FIH) studies and to...
How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of...
Can CYP3A4 Induction Predict P-glycoprotein Induction in DDI Studies?
- Enzyme Induction
- August 20, 2019
- Shanté Jackson, Madison Esely-Kohlman
Generally, a drug’s effects on enzyme and transporter activity are examined independently in a drug development program, but what if...
In Vivo ADME: What You Need and Why You Need It
- In Vivo & Radiolabeling
- July 22, 2019
- Madison Esely-Kohlman, Satoshi Ito
When putting together a data package for regulatory approval by the FDA, EMA, or PMDA, there is a lot to...
Cell Handling & Media Selection for Best Results in Hepatocyte Assays
- Test Systems & Methods
- June 19, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Getting reliable, reproducible results from studies using hepatocytes as a model system for drug metabolism is in part dependent on how well...
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback