Author: Dr. Brian Ogilvie
FDA Guidance: Many In Vitro DDI Evaluations Should Precede FIH Studies
- Regulatory Guidance
- December 6, 2018
- Dr. Brian Ogilvie, Andrea Wolff
In October 2017, the FDA released its much-anticipated draft guidance documents for drug-drug interaction (DDI) studies, which was finalized in...
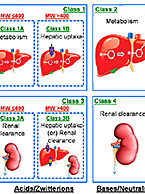
New Recommendation: Evaluate NMEs for OCT1 Transporter-Mediated DDI Potential
- Drug Transporters
- October 1, 2018
- Dr. Brian Ogilvie, Andrea Wolff
Brian Ogilvie, Ph.D., Vice President of Scientific Consulting at XenoTech, presented a case study on the importance of the hepatic OCT1...
New FDA Draft Guidance “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics” – XenoTech’s perspective on in vitro DDI testing
- Regulatory Guidance
- July 11, 2022
- Dr. Maciej Czerwinski, Dr. Pallavi Limaye, Dr. Brian Ogilvie
The FDA has released a new draft guidance for industry titled “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics.”...
DDI & Drug Repurposing Article featured in Drug Discovery World (DDW) Spring Edition 2021
- Drug Drug Interactions (DDI)
- April 29, 2021
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Repurposing (repositioning, re-profiling, or re-tasking) a drug potentially saves years of costly testing from going to waste and potentially providing a higher chance of success.…
What is ADME and how does it fit into drug development?
- Drug Metabolism
- April 10, 2020
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The main aim of drug development is to get a compound that has a therapeutic effect into the form of...
Transporters of Emerging Importance in Drug Development: Beyond the Guidance Documents
- Regulatory Guidance
- June 25, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
Dr. Ogilvie’s presentation discusses critical literature and case studies which have been published following the FDA’s 2017 guidance revision, and covered...
Official PMDA English Translation
- Regulatory Guidance
- May 7, 2019
- Madison Esely-Kohlman, Dr. Brian Ogilvie
The Japanese regulatory agent PMDA (Pharmaceuticals and Medical Devices Agency) recently published an official English translation of their final Drug Interaction Guideline...
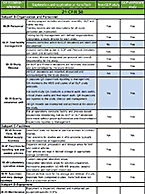
To GLP or not to GLP?
- Regulatory Guidance
- October 5, 2018
- Scott Hickman, Dr. Brian Ogilvie, Tim Patterson
That is the question. . . Knowing the answer may save you time and money Good Laboratory Practices (GLP) are...
IQ Induction Working Group Publishes Recommendations for Data Interpretation of In Vitro Induction – Focus on CYP3A4 mRNA
- Enzyme Induction
- July 13, 2018
- Scott Hickman, Dr. Brian Ogilvie
XenoTech is proud to have made contributions to the paper entitled, “Considerations from the IQ Induction Working Group in Response...
Initial Impressions of New Draft FDA DDI Guidance Documents from XenoTech
- Regulatory Guidance
- October 26, 2017
- Michael Millhollen, Dr. Brian Ogilvie
Updated Nov. 6th, 2017 The FDA has released its long-awaited new draft guidance for industry on drug-drug interaction (DDI) studies....
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback