

Cell Handling & Media Selection for Best Results in Hepatocyte Assays
- Test Systems & Methods
- June 19, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Getting reliable, reproducible results from studies using hepatocytes as a model system for drug metabolism is in part dependent on how well you store and handle the cells. Hepatocytes require more finesse than other cell types, and some considerations and precautions in handling might not be obvious to scientists that are new to performing studies using cryopreserved, primary hepatocytes, but have experience with cell lines. Additionally, making the right choices with respect to media is key to seeing the best results in viability, attachment, and culture life of your cells. Here we discuss some tips for handling hepatocytes and making the most of your media:
1. Choose your hepatocytes wisely
XenoTech is well-known as a hepatocyte provider with top-tier quality and lot variety to meet specific needs of drug developers and academics. We know what’s important to our customers in what they look for: do the hepatocytes have high CYP activity? Do they attach to the surface of the well? Do they form a confluent monolayer? Do they have >70% post-thaw viability? Our 25 years’ experience has led to a superior in-house production team that isolates cells from donor tissue with the efficiency and skill critical to high quality hepatocytes, and a QC team that tests every lot to ensure our customers get the best quality data.
2. Transport your cells carefully
Hepatocytes are very temperature sensitive! If you keep your cells in a storage area separate from the lab in which preparation and testing is performed, use a liquid nitrogen Dewar instead of dry ice pellets to ensure that cells do not undergo temperature fluctuations while transporting over a significant time/distance—from the moment hepatocytes exit the controlled cryo-freezer, time is precious! Improper transport can reduce viability by 30%; don’t let transport time affect your samples before you’re ready to prep.
3. Minimize freeze-thaw cycles
Each time cryopreserved hepatocytes are thawed and refrozen, as in the case of creating pooled human hepatocyte lots from previously cryopreserved donors, those hepatocytes sustain cryoinjury which will reduce viability and metabolic activity of cells. Choosing single-freeze pooled hepatocytes is a good way to avoid unnecessary damage. CryostaX technology offers features not available from the traditional vial format: customizable yield, unprecedented large lots, and minimal cryoinjury during pooling, all while only having undergone a single freeze thaw cycle. With CryostaX, viable cellular assured minimum yield (AMY) per vial of individual human donors and/or rodent hepatocytes can be adjusted up or down without having to undergo additional cryoinjury. Also, because pellets do not need to be thawed and refrozen to combine lots, we can offer unprecedented large rodent lot sizes which could offer customers lot consistency for years. With regards to human pools, CryostaX affords us the unique ability to quickly create standard pools (donor number can be anywhere from 2 to 10) and/or custom pools based on customer specified demographics and characteristics, all without having to use a detrimental second freeze-thaw cycle. An additional benefit is a simplified thaw process. When using conventional cryopreserved samples you need to thaw vials in a water bath before transfer to media, but CryostaX pellets can be delivered from the vial straight into pre-warmed media, saving time and preventing damage from over-thawing. Current CryostaX selection includes pooled rodent suspension or plateable hepatocytes and pooled and individual human hepatocytes—and we are expanding to additional species in the near future!
4. Use optimized media
While media products from different providers might fit the same description, minute differences in formula can make a big difference in how well hepatocytes thrive in culture. Our experts use our products in all services carried out in-house, and have many studies’ worth of data to optimize our media formulas.
Our products team is committed to updating our product offerings to optimize for quality and ease of use. For example, as of June we have introduced a new Rodent CryostaX thawing media formula, OptiThaw K8800. The new formula extends compatibility to all rodent CryostaX hepatocytes, maximizing viability of hepatocytes post-thaw while expanding application options.
Below is a quick product guide for use in each stage of preparing hepatocyte cultures:
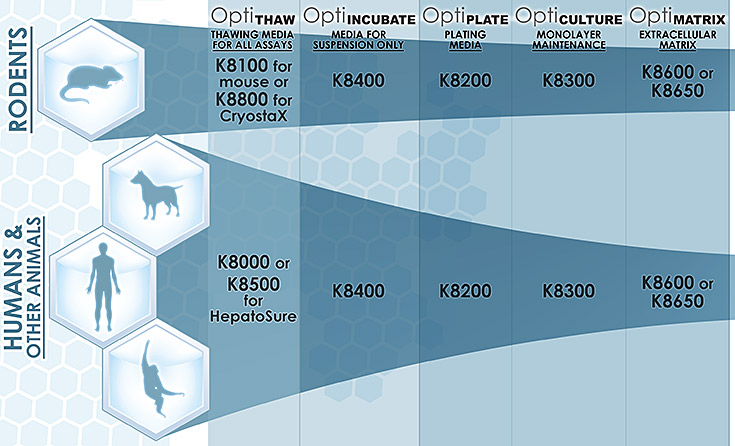
Product Name: OptiThaw
| Product Number | Use | Features |
|---|---|---|
| K8500 – for HepatoSure |
Thawing media
|
Short spin time (5min) compared to competitors (7-15 min)
|
| K8100 – for mouse hepatocytes |
Thawing media
|
One vial of media can be used for up to three vials of hepatocytes at one time
|
|
Thawing media
|
Online how-to video demonstrating proper thawing procedure
| |
| K8000 – for all other hepatocytes |
Thawing media
|
Comes with ready to use Trypan blue counting tubes to accurately determine viability and yield
|
Product Name: OptiPlate
| Product Number | Use | Features |
|---|---|---|
| K8200 – all species | Plating media | Special mix of growth factors optimize hepatocyte attachment
Standardized for all species For plateable hepatocytes |
Product Name: OptiMatrix
| Product Number | Use | Features |
|---|---|---|
| K8600 – .5 mL | Extracellular matrix | Proprietary mix of supplements to help maintain cells in culture ECM overlay for application 3-4 hours after plating Helps re-polarize cells |
| K8650 – 1.5 mL | Extracellular matrix | 2 convenient sizes for different well formats Maintains cell morphology and integrity of monolayer Increase confluency |
Product Name: OptiCulture
| Product Number | USe | Features |
|---|---|---|
| K8300 – all species | Monolayer maintenance | Serum-free
Standardized for all species For plateable hepatocytes Maintains culture stability for at least 5 days |
Product Name: OptiIncubate
| Product number | Use | Features |
|---|---|---|
| K8400 – all species | Hepatocyte Media | For suspension hepatocytes
Optimized for 4-6 hour incubation time Serum-free |
5. Keep it moving
After you’ve thawed and resuspended your hepatocytes in media and you’re ready to plate, make sure that you are keeping the cells in suspension; hepatocytes tend to settle to the bottom of the vial so without proper mixing, one micropipette full of solution might contain more or fewer cells than the next. Consistency in plating is key to accurate counts and uniformity between plates for better comparison. See our how-to video as a guide for best practice.
6. Be aware of myths
Our products team has vetted every component that makes up our media to create balanced formulations that keep cells at top functionality for as long as possible to make sure you get dependable results.
Our media products are relatively new to market; protocols written before there was much diversity in options may specify a particular brand, but that doesn’t mean it is still the best out there today! We encourage our customers to consider that every choice along the way should be made with careful consideration to maintaining hepatocyte health. The media formulas we have developed reflect countless hours of experience our research scientists have put in using our products to conduct the same studies our customers use them for.
Our team works every day to bolster our hard-earned reputation for providing consistent, high-quality hepatocytes to customers, and have applied our incomparable expertise to develop superior complementary products – that we ourselves use for contracted studies! – to make sure you have the best tools available to yield dependable, quality data.
Learn more about:
About the Authors
Related Posts
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
