

Why Switch to Hepatocyte Pellets?
- Test Systems & Methods
- June 30, 2021
- Michael Millhollen, Dr. Chris Bohl
You may have heard about the patented CryostaX® format of hepatocyte pellets, but do you know why XenoTech and countless other researchers have made the switch to pellets from traditional hepatocyte preparations? The reasons vary depending on who you ask, from the needs of the study to simply convenience, so we’re going to outline the primary factors and let you decide for yourself.
Pooling: Maximize Metabolic Activities by Limiting Hepatocyte Cryoinjury
Cryopreservation and thawing of primary hepatocytes causes inherent damage to the cells. Traditional pooling methods require cells to undergo two separate cryopreservation and thaw steps, leading to increased cryoinjury and decreased metabolic activity.
The patented CryostaX® hepatocyte cryopreservation process eliminates one of those steps. Individual donor hepatocytes are cryopreserved into CryostaX® pellets instead of to the inside of a vial. Those pellets can then be easily combined in any combination without having to thaw, mix, and re-cryopreserve a second time.
As you can see in a poster that we presented at the 9th International ISSX Meeting in 2010, data indicates that minimizing cryoinjury leads to a modest conservation of CYP450 and SULT activities and has significant benefits to UGT activity. So why choose hepatocytes with unnecessary cryoinjury?
Pooling: Fast No-Cost Customization of Human Hepatocyte Pools
As mentioned above, single-donor CryostaX® hepatocyte pellets can be combined into customized pools very easily, and easy means quickly at no additional cost. Once the proposed pool is approved by the customer, the pool can be typically made and out the door within one and a half weeks. This allows scientists to create pools with up to 20 donors in a standard vial based on the specific needs of their study. Each individual donor is extensively characterized, so custom pools can be designed based on donor demographics, medical histories, genotyping, metabolic activity and more. Common options include:
- Specific Enzyme Activity
- Pool Size
- BMI
- Disease State
- Age, Ethnicity &/or Gender
- Alcohol, Tobacco &/or Drug Use
- Serologies
Use with Microsome Stable, Slower Turnover Compounds
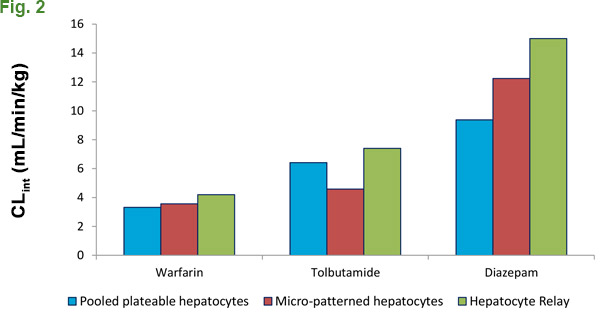
Compounds that exhibit high metabolic stability in suspension hepatocytes and subcellular fractions (S9, microsomes and cytosol) can be a challenge for ADME scientists, as they are traditionally limited to an incubation window of up to 6 hours depending on the test system, taking advantage of the time when they are the most metabolically active and can generate quality data. As previously mentioned, the absence of the second freeze/thaw step when pooling results in less cryoinjury. Less cryoinjury leads to higher quality cells. When used with XenoTech’s optimized medium and protocol, plateable CryostaX human hepatocytes can be cultured for up to 48 hours without a medium change, giving a significantly extended incubation window in which to collect metabolism data (Fig. 1). We successfully used plated hepatocytes to measure substrate loss and calculate intrinsic clearance, even for notoriously low-turnover small molecule drugs. When compared to alternative methodologies used to assess low-turnover compound metabolism, CryostaX® generates comparable metabolism data in a format that is easier to use and more cost effective. (Fig. 2). Discounted test vials are available to evaluate and qualify specific lots for this purpose if you have an applicable compound.
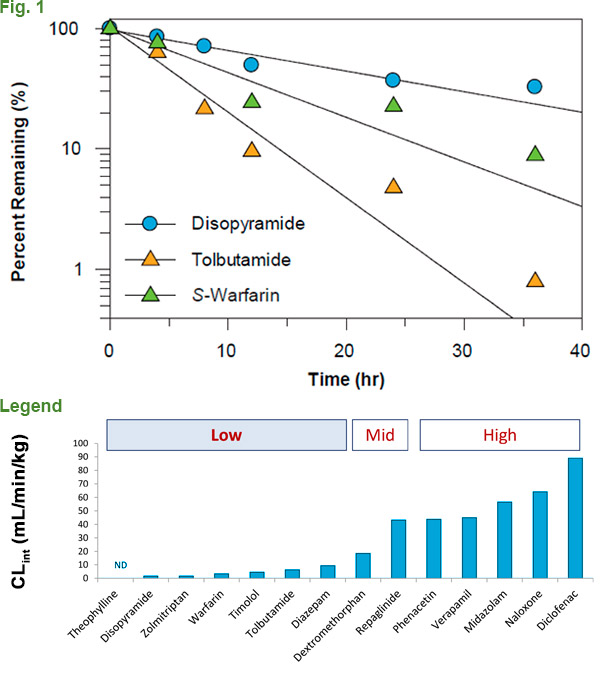
Legend. Clint values for 14 compounds with CryostaX® pooled plateable hepatocytes.
Easier to Use and Reduced Risk of Contamination
CryostaX® hepatocytes also eliminate the need for water baths that are traditionally required to thaw cryopreserved hepatocytes and release them from the vial. By removing water baths in or near culture hoods and incubators, a significant source of tissue culture contamination can be decreased or even eliminated. CryostaX® hepatocytes can be taken directly from nitrogen storage and simply poured into pre-warmed thaw medium. Watch our Hepatocyte Thawing Demo Video to see them in use.
In addition to negating potential contamination, researchers have stated that they enjoy the increased efficiency and performance consistency from eliminating the thawing step. As there is no longer a danger of cell damage from over-thawing, customers have expressed increased confidence in using CryostaX® hepatocytesbecause of reduced or eliminated variability due to differences in user skill levels and experience.
And all of the benefits above are just the beginning. Other notable features of CryostaX® are:
- The only single-freeze, pooled plateable human hepatocyte product on the market
- Reduce costs by customizing Assured Minimum Yield (AMY) to only what you need
- Pooling pellets leads to each donor contributing more equally to the pooled population and metabolic activity
- Pooled lots are extensively characterized for metabolism and uptake transport, with CYP induction (10-Donor Attaching Pool)
- Highly competitive pricing
- Large lot sizes
So even when researchers don’t have specific study requirements that would benefit from CryostaX®, they’re still choosing to switch to a pellet format for the other benefits. Here are a few comments that we’ve received from CryostaX® customers:
“We have used 2 different lots of Human 10 donor pooled CryostaX and overall the viability has been very good. Typically I have observed close to or above the noted viability from the product specification sheets. In addition the yields per vial have been consistent to the product sheets. What is most helpful are the enzyme activity numbers as our in vitro clearance results match well with activity results provided by XenoTech. The CryostaX pellets were very easy to work with as there was no need for a thawing step.”
“I found the CryostaX very easy to work with and I’m definitely comfortable using them. The onsite training we received was excellent but I think the video and protocol would be sufficient for many users.”
“Whoa, I love the cryo pellets. That really takes the ‘art’ out of thawing. You guys should do that with all of your cryo, I’m not kidding.”
Comments like that are why we have expanded our CryostaX® options beyond individual-donor and pooled human hepatocytes for suspension or plating. We also offer monkey, mouse and rat hepatocyte pellets and will continue adding more options to the CryostaX® lineup in the future.
Dr. Chris Bohl, our Global Technical Support Manager for Products, commented, “We’re excited about the convenience our CryostaX hepatocytes offer for our customers. We have hepatocytes available from numerous species in many formats to fit the needs of different types of research, but the patented, unique format of CryostaX is truly a game-changer for the increased efficiency, unparalleled flexibility, and greater utility they provide for drug development.”
So take a look at our online cellular product listings to see if any of our currently available lots match your needs, contact us with any questions or requests, and don’t forget to sign up for our newsletter – new CryostaX® lots often sell pretty quick. You can also use the form below to submit a custom CryostaX® pool request:
About the Authors
Related Posts
New Rodent, Monkey CryostaX Hepatocytes Featured in Industry News
How to Choose the Right Test Systems for Your DMPK Studies
Subscribe to our Newsletter
Stay up to date with our news, events and research
Do you have a question or a request for upcoming blog content?
We love to get your feedback
