

How to Choose the Right Test Systems for Your DMPK Studies
- Test Systems & Methods
- September 12, 2019
- Dr. Chris Bohl, Madison Esely-Kohlman
Test systems for DMPK in vitro studies are part of the very foundation of our company. Our labs were borne of the rare expertise of our founders working with hepatocytes and subcellular fractions to grow scientific knowledge and methods central to drug metabolism, and we haven’t stopped pushing the limit since. XenoTech is one of the largest producers of tissue-derived in vitro products in the world, and we didn’t get there by accident. Our teams work around the clock to prepare products suited to get the very best data for DDI and ADME assays—our services teams use the same products we provide to customers, a testament to our commitment to quality.
We also draw upon this experience when guiding our customers to the right test systems for what they need to accomplish. There are myriad options for what materials to use for which assays, but in our vast experience we have compiled some rules of thumb for how to pick the right test systems for you.
Hepatocytes
Primary hepatocytes are often referred to as the “gold standard” for in vitro hepatic assays. The majority of hepatocyte-specific cellular function can be maintained in culture, which makes them the best model for replicating liver function in vitro, e.g. xenobiotic metabolism, hepatotoxicity, and host-pathogen interactions. In drug development, hepatocytes are best suited for metabolism, clearance, uptake, hepatotoxicity, and drug-drug interaction assays. They are a good model because in context of biotransformation most drug metabolizing-enzymes are abundantly found in this particular type of cell in the human liver, one of the primary sites where drugs are metabolized. Primary hepatocytes therefore serve as a convenient and cost-effective alternative to in vivo testing.
There are options for what format is best suited for each DMPK assay. Researchers looking for primary hepatocytes are offered choices between cryopreserved or fresh, plateable or suspension, and individual or pooled—how to choose?

Cryopreserved vs fresh
Cryopreserved hepatocytes are most common and easy to work with because the cryopreservation process enables long-term storage while conserving function; access to the same lot can provide consistency in results from tests performed at different times, whereas using fresh hepatocytes necessitates immediate use and no opportunity to use cells from the same isolation in later assays. Upon thawing, cryopreserved hepatocytes are shown to maintain clinically significant activity levels and plateable lots are validated for ability to attach and form a confluent monolayer on collagen substratum.
Scientists on the XenoTech product development team have patented what we believe to be the best format for cryopreserved hepatocytes: Our CryostaX are essentially pellets, each pellet composed of hepatocytes from an individual donor. This format allows for individual donor preparations, consistency with regards to post thaw yield between lots, large pooled lot sizes, one-step thawing to minimize cryoinjury, as well as the benefit of easily customized pools by combining pellets of donors selected for age, demographic, disease-state characteristics, etc.
We also offer fresh hepatocytes. Due to the time-sensitive nature of working with fresh hepatocytes, we encourage you to sign up for our Liver Alert system, which sends an email notification when fresh human hepatocytes become available. To learn more about this service or to sign up, visit xenotech.com/liver-alert or contact our customer service office at 913-GET-P450. Fresh animal hepatocytes can be scheduled or prepared upon request.
Plateable vs suspension
We offer 2 main categories, with multiple sub groups, of cryopreserved primary hepatocytes:
Cryoplateable hepatocytes are the closest alternative to fresh hepatocytes, and are used most often in confluent monocultures with an overlay of extracellular matrix, which best replicate in vivo conditions. This format allows drug developers to study hepatobiliary drug transport, metabolism, drug-drug interactions, and hepatotoxicity.[1]
Our plateable hepatocytes can be used in monoculture or coculture formats; while monoculture is best suited for observing activity of enzymes abundant in hepatocytes, cocultures incorporate other cell types and can be used in 2D or 3D systems.
Our cryoplateable animal and human hepatocytes are qualified by their ability to maintain confluent monolayer for at least five days (critical for induction studies and metabolism studies of low-turnover compounds), and human lots can be further characterized for specific CYP activity or general UGT and SULT activities. For user convenience, each lot is also tested for optimal seeding density, noted on the accompanying datasheet.
Hepatocytes in suspension are used primarily for compound stability, metabolite formation, extended incubations using the relay method, inhibition studies, uptake, and gene expression studies. Suspension lots are best suited for short-term studies (4-6 hours) due to decreases in viability and metabolic activity over time.
Individual or pooled
You also have the option in either format to select individual donors or pooled lots. Single donor lots are necessary for some definitive preclinical studies, such as induction studies, while in other studies pooled lots provide consistency and wider representation due to larger sample size.
Pooled hepatocytes can be used for the majority of the assays that individual donor hepatocytes are commonly used for, with fewer concerns regarding inherent donor variability. Pooled donor hepatocytes can be used for induction screening, but aren’t typically used for definitive induction studies where data is used to apply for an IND. Human hepatocyte pools can be easily customized for number of donors (usually up to 10 for attaching and 20 for suspension) and phenotype criteria (race, age, gender, BMI, diabetic, alcohol and drug use, etc.).
We also offer our HepatoSure 100-donor pool, which provides you with advantages such as better enzyme activity averages, long-term availability of the same pooled product for lot-to-lot consistency, and better representation of the average population.
When using primary human hepatocytes, observing proper procedure in thawing and handling the cells is crucial as they do not propagate in culture and any damage that sends a cell towards apoptosis/necrosis early in the thawing and plating process will almost always lead to disappointing results. You can read more about storage and handling considerations here.
Subcellular fractions
Subcellular fractions are widely used as a test system in early stages of drug development for metabolism and screening studies. Fractions can be obtained by successive centrifugation from any tissue and are very easy to work with because incubations require none of the time, extensive training, and resources of cell cultures. An additional benefit is suitability for full automation and data can be generated quickly. Our catalogue of subcellular fractions is extensive and options for subcellular products encompass pools of variable donor characteristics. Subcellular fractions include matrices appropriate for use in metabolic stability, metabolite ID, enzyme inhibition, reaction phenotyping, species differences, and metabolite formation.
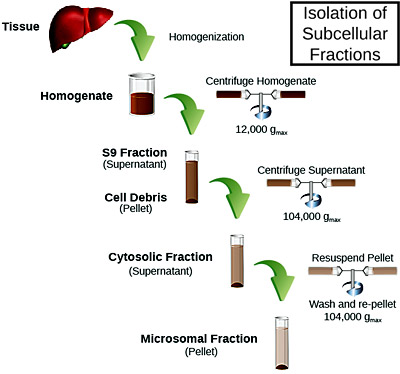
Microsomes
Human liver microsomes are the most widely used in vitro model for drug metabolism for the ability to predict interaction with major drug-metabolizing enzymes.[2] Microsomes are subcellular fractions containing high specific activity of membrane-bound phase I metabolic enzymes. High concentrations of these enzymes, including CYPs and UGTs, make microsomes an ideal test system for species comparisons, metabolic stability, inhibition, and reaction phenotyping studies during discovery and lead optimization. Due to the small size of microsomes, a large donor pool can be used in an assay which can be instrumental to represent enzymatic activity levels expressed in the general population, and ease of use makes them ideal for high-throughput assays.
S9
S9 fractions are a more complex mixture of enzyme activities and is more representative of whole cells/ tissue than microsomes, as they contain both microsomal and cytoplasmic enzymes. Cytoplasm contains some phase II enzymes not found in microsomes, which may be important to consider if your drug candidate is predicted to be metabolized by these enzymes.
Ability to optimize microsomal and S9 test systems is key to achieving relevant, useful data. Our highly-practiced team and access to well-characterized source tissue makes it possible for us to provide subcellular fractions from many different species and organs, and in the case of human liver microsomes, categorized further for donor number, donor gender, disease state, or genotype. Having these options available helps DMPK scientist select the test system most representative of their target population and can therefore be more predictive of in-patient conditions.
Lysosomes
Purified lysosomes and tritosomes (lysosomes isolated from animals treated with Tyloxapol for improved separation of the organelle from mitochondria) are valuable tools for investigating compound stability of some drugs. Many of the most common enzymes involved in the degradation or catalysis of oligonucleotides, peptides, nanoparticles, antibodies, ADCs, and other biopharmaceuticals are contained in the lysosome and studies using this test system are helpful in understanding metabolic stability of compounds that are trafficked through the cellular endocytic/lysosomal pathways.
Recombinant enzymes
Recombinant enzymes are the simplest system available to study enzyme interaction. Different isoforms of CYPs and UGTs are engineered to enable researchers to distinguish a xenobiotic’s effect on activity of individual enzymes. Some enzymes, such as CYP3A4 and CYP3A5, are very similar in structure and are hard to distinguish in LC/MS analysis. Recombinant enzymes, therefore, are most often used to confirm any ambiguity observed in reaction phenotyping studies. In cases of low metabolism, recombinant enzymes can be used at high concentrations to amplify metabolic activity for use in metabolic stability screening and inhibitory potential evaluation.[3] Both recombinant CYPs and UGTs can be helpful for these applications, and through Cypex we are excited to offer a wide selection of recombinant human enzymes—offerings can be seen here.
Tissue
Efforts to develop treatment options for liver disease such as NAFLD, especially NASH, have been rapidly growing for the last few years and research on diseased liver function has been assisted in part by whole tissue products. We offer multiple forms of snap frozen liver tissue, such as prelysate tissue frozen in buffer, cryopulverized, embedded and sectioned, and disease-specific tissue microarray (TMA). Among the various formats, healthy tissue is available as well as samples representative of steatohepatitis, steatosis, cirrhosis, and diabetes from donors with or without histories of alcohol use. Additionally, in response to the need for better tools to make strides in treating children affected by liver disease, pediatric tissue is available, as well. Tissue products are used in various applications including transcriptome and proteome arrays, genotyping, biomarker confirmation, and identification of drug target candidates.
Cell bank
Immortalized cell lines are used in many fields of research even beyond drug development and disease progression. The Japanese Collection of Research Bioresources (JCRB) is a bank containing more than 1,200 cell lines with unique qualities including cancer, mutant, genetically-modified, and immortal cells. You can use the Cell Search database on the JCRB website to search for appropriate cell lines by animal, tissue, morphology, cell type, or classification. North American and EU clients can work directly with XenoTech representatives to streamline the order process, making it easy for clients to order their JCRB cell lines without having to worry about shipping and customs.
Custom Test Systems
While there are many commonly used test systems that are readily available, we also understand that not every need can be met by standard products. We fill in the gaps by dedicating a special Custom Products team with access to a wide array of species, tissues, fractions, and enzyme/transporter activity characterization methods to help you obtain the perfect test system for your DMPK needs. To see just a few examples of what is possible, please see our custom test systems page.
Learn more about:
References
- https://www.ncbi.nlm.nih.gov/pubmed/20109035
- https://www.sciencedirect.com/referencework/9780080450445/comprehensive-medicinal-chemistry-ii
- https://www.ncbi.nlm.nih.gov/pubmed/16248842
About the Authors
Related Posts
Learn More
View a complete list of XenoTech’s products as well as their uses and applications

Contact Us
with questions or feedback

