

Spotlight on Efflux and Uptake Drug Transporters in In Vitro Drug-Drug Interaction Studies
- Drug Transporters
- June 23, 2022
- Dr. Andrew G. Taylor, Michael Millhollen, Isis Smith
What are drug transporters?
Drug transporters are membrane-bound proteins that assist in the movement of drugs into or out of cells. Drug transporters are found in all cells throughout the body, but drug developers and ADME scientists are mainly concerned with transporters located in the liver, intestines, kidneys and the blood-brain barrier when considering drug-drug interactions (DDIs).
Drug transporters can be split into two superfamilies. The first are the ATP-binding cassette (ABC) transporters, also known as efflux transporters. Efflux transporters use energy released from the hydrolysis of ATP to actively move compounds from inside to outside the cell. The main efflux transporters are:
- P-gp (MDR1)
- BCRP
- BSEP
- MRPs
The second superfamily of transporters are the solute carrier (SLC) transporters, also called uptake transporters. These transporters do not require ATP and are responsible for moving compounds from outside to inside the cell. Some of the main uptake transporters are:
- OATP1B1/3
- OCT1/2
- OAT1/3
- MATE1/2K
Transporter Expression and Function
The focus on specific transporters generally depends on which tissue are being evaluated. For example, when considering the intestine and blood-brain barrier, efflux transporters – especially P-gp and BCRP – are important because they regulate the absorption, or entry, of drugs into the brain or into the cells of the intestine. Efflux transporters are also involved in pumping compounds into the bile or urine for excretion. When looking at the liver or kidney, uptake transporters are important to consider because they transport compounds from outside to inside of the cell.
Why are drug transporters important?
Drug transporters can be thought of as the A, D, and E in ADME. They are responsible for:
- Absorption
- Distribution
- Tissue-specific drug targeting
- Elimination of compounds
Since drug transporters are so involved in the movement of drugs and compounds, they can be involved in many DDIs. The clearance of transporter substrates (Victims) can be impacted by transporter inhibitors or inducers (Perpetrators) and this can lead to toxicity or loss of efficacy of a given drug.
One example of this is statins. Statins perform their function in the liver and require OATPs to enter into hepatocytes. Cyclosporin is a potent inhibitor of OATP transporters. Therefore, if you’re taking cyclosporin and statins at the same time, you can see a large increase in statin exposure. This can lead to toxic side effects such as rhabdomyolysis, where the muscles break down and release their contents into the bloodstream, which can eventually lead to kidney failure and possibly death.
Regulatory Guidance on Transporters
The regulatory guidance has very clear recommendations when evaluating transporters for drug-drug interactions. The FDA, EMA, and PMDA generally overlap for the 9 main transporters that should be investigated. These include the efflux transporters P-gp and BCRP along with uptake transporters OATP1B1/1B3, OAT1/3, OCT2, and MATE1/2K. Regulatory guidance also recommends that other transporters should be considered as necessary, including BSEP, MRP2, and OCT1.
All of the regulatory agencies recommend that P-gp and BCRP substrate potential should be evaluated. The substrate potential for other transporters should also be considered based on the clearance/elimination routes and/or knowledge of other drugs that are in the same therapeutic class. For example, if the clearance of the compound is 25% or greater through a given elimination pathway, the transporters within that elimination pathway need to be considered for substrate potential of the compound.
For inhibitor evaluation, the FDA guidance for industry recommends that 9 main transporters be investigated, while the EMA recommends 11. In addition, the induction of the transporter P-gp should be considered if the compound is a known inducer for CYP3A4 or for the PXR or CAR pathways. Lastly, any metabolites of the parent drug need to be considered for potential transporter interactions if the metabolite is 25% or more of the parent drug AUC.
In Vitro Drug Transporter Studies
At XenoTech, our definitive transporter studies are designed to meet the requirements of the FDA, EMA and PMDA regulatory agencies. Concentration of solvents is kept low (generally at 0.1%) and going higher than 1% of solvent is avoided. Preliminary experiments that are recommended by FDA guidance, including cytotoxicity, stability of the compound in media, and non-specific binding of the compound to the experimental plates, are included in all definitive study designs. Transporter studies are also offered in a slimmed down, medium-throughput screening (MTS) study design that is useful for lead compound selection.
Drug Transporter Inhibition
The general design of our transporter inhibition studies involves:
- Keeping the probe substrate concentration much lower than the Km
- Using a short incubation time to keep within linear range while still obtaining acceptable fold uptake of the probe substrate
- Keeping the test article at the highest concentration based on EMA and FDA guidance recommendations while still considering cytotoxicity and solubility limitations
Our definitive design experiment includes 7 concentrations of the test article plus a no-solvent and solvent control in order to determine IC50 should any inhibition be observed. Additionally, we offer a minimal design experiment, which is a MTS design that has 2 concentrations of the test article along with the no-solvent and solvent control. All experiments include the appropriate positive controls with the specific positive control substrates and inhibitors.
Transporter Substrate Potential
Our transporter substrate assays keep the concentration of test article at a pharmacologically relevant concentration, ensuring the concentration is not so low that the compound is below the limit of quantitation or so high that it saturates the transporter, as either of these conditions can make the evaluation of substrate potential difficult.
Definitive study designs include 4 concentrations of test article with 3 time points, and include treatments with or without inhibitor along with the appropriate positive controls.
General Transporter Study Designs
We have three basic designs of our experiments, which depend on the transporter that’s being evaluated:
- ABC Transporter: Efflux Transwell Assays
- SLC Transporter: Uptake Assays
- Vesicles Assays
ABC Transporters: Efflux Transwell Assays
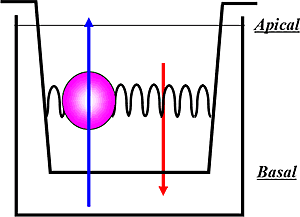
The transwell assay can be thought of as a cup within a cup with a polarized monolayer of cells grown in between them, as represented in the diagram above. This creates an apical (A) section and a basal (B) section on either side of the monolayer of cells. Compound is added either to the apical side or the basal side and the efflux of the compound in either direction is measured. The transwell assay format is used for evaluating both ABC transporter inhibition and substrate potential.
For efflux transporter substrate assays, the bidirectional permeability of the test article across the cells in both the A to B and B to A directions is measured; while for efflux transporter inhibition assays, the effect of the test article on the bidirectional permeability of a known probe substrate for the given transporter is measured.
| Transporter | Probe substrate | Positive control inhibitors |
|---|---|---|
| P-gp | Digoxin | Valspodar, Verapamil |
| BCRP | Prazosin | Ko143, Lopinavir |
The MTS cell permeability assay can be conducted with Caco-2 or a definitive study can be conducted in either Caco-2 or MDCKII cells.
SLC Transporters: Uptake Assays

Uptake transporter assays are used for evaluating both SLC transporter inhibition and substrate potential. For uptake transporter substrate assays, the accumulation of the test article in transfected and control cells measured. For uptake transporter inhibition assays, the effect of the test article on the accumulation of a known probe substrate for that given transporter is measured. Once again, all experiments are conducted with the appropriate known probe substrate and positive control inhibitors.
| Transporter | Probe substrate | Positive control inhibitors |
|---|---|---|
| OATP1B1/ OATP1B3 | 3H-Estradiol-17β-glucuronide | Rifampin, Cyclosporine |
| OATP2B1 | 3H-Estrone-3-sulfate | Erlotinib, Bromosulfophthalein |
| OAT1 | 3H-p-Aminohippurate | Probenecid, Novobiocin |
| OAT3 | 3H-Estrone-3-sulfate | Probenecid, Ibuprofen |
| OCT2 | 14C-Metformin | Quinidine, Cimetidine |
| OCT1 | 14C-Tetraethylammonium bromide | Quinidine, Verapamil |
| MATE1/2K | 14C-Metformin | Cimetidine, Pyrimethamine |
Vesicle Assays
Vesicle assays are also used when evaluating inhibition and substrate potential of efflux transporters. Additionally, they are the preferred test system for studies to investigate the kinetics of efflux transporters. Vesicle assays can be used to evaluate P-gp, BCRP, MRP1, MRP3 and MRP4, along with BSEP and MRP2. The design of the vesicle assay is similar to that of the uptake design; however, it includes treatments with and without the co-factor ATP.
| Transporter | Probe substrate | Positive control inhibitors |
|---|---|---|
| BSEP | 3H-Taurocholate | Cyclosporine, Glyburide |
| MRP2 | 3H-Estradiol-17β-glucuronide | Benzbromarone |
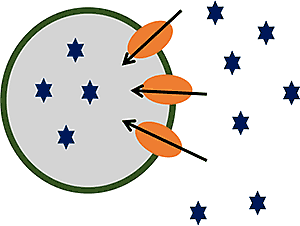
Vesicles are small, inverted plasma membranes derived from cells that over express the transporter of interest. For vesicle substrate assays, the accumulation of test article inside the transfected vesicles is measured in the presence and absence of ATP. For inhibition assays, the effects of the test article on the accumulation of a known probe substrate in the presence and absence of ATP is measured.
Transporter Results Example Data: P-gp Inhibition Experiment

In this example data, the y-axis shows the different concentrations of the test article ranging from low to high, along with no-solvent and solvent. The x-axis shows the apparent permeability (Papp) of digoxin in the basil to apical direction with the dark bars, and the apical to basal direction with the light bars.
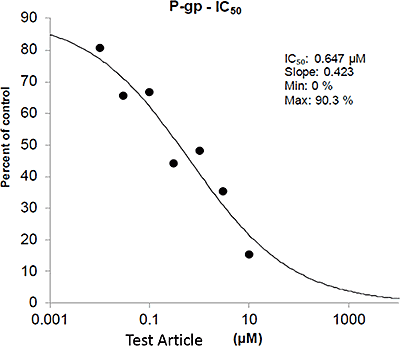
By slightly rearranging this data then looking at the efflux ratio of digoxin and comparing it to the efflux ratio of the solvent control, an IC50 can be estimated. This second graph is the same data from the first graph, but rearranged so the y-axis shows the percent of control of the efflux ratio of digoxin as a function of increasing concentrations of test article.
Supported Transporters and Related Test Systems at XenoTech
Depending on the different routes of elimination or absorption of your drug, you might be interested in testing other transporters aside from the 9 main transporters that are recommended by the regulatory agencies. XenoTech and our partners can perform studies for numerous other transporters that you may want to consider, so please contact us to discuss your study needs.
In addition to the many drug transporter services that we offer, we also provide a number of products that you can purchase to conduct uptake transporter studies within your own lab. These include test systems such as:
- Primary human hepatocytes for suspension
- Primary human hepatocytes for plating
- Pre-plated human primary hepatocytes
- Primary animal hepatocytes for suspension
- Primary animal hepatocytes for plating
- Pre-plated animal primary hepatocytes
We also offer numerous support reagents to go along with these hepatocytes. If you have any questions or requests, please feel free to contact us to be put in touch with our expert team. We also have numerous other resources on drug transporters and other relevant topics.
About the Authors
Related Posts
Subscribe to our Newsletter
Stay up to date with our news, events and research

Do you have a question or a request for upcoming blog content?
We love to get your feedback
